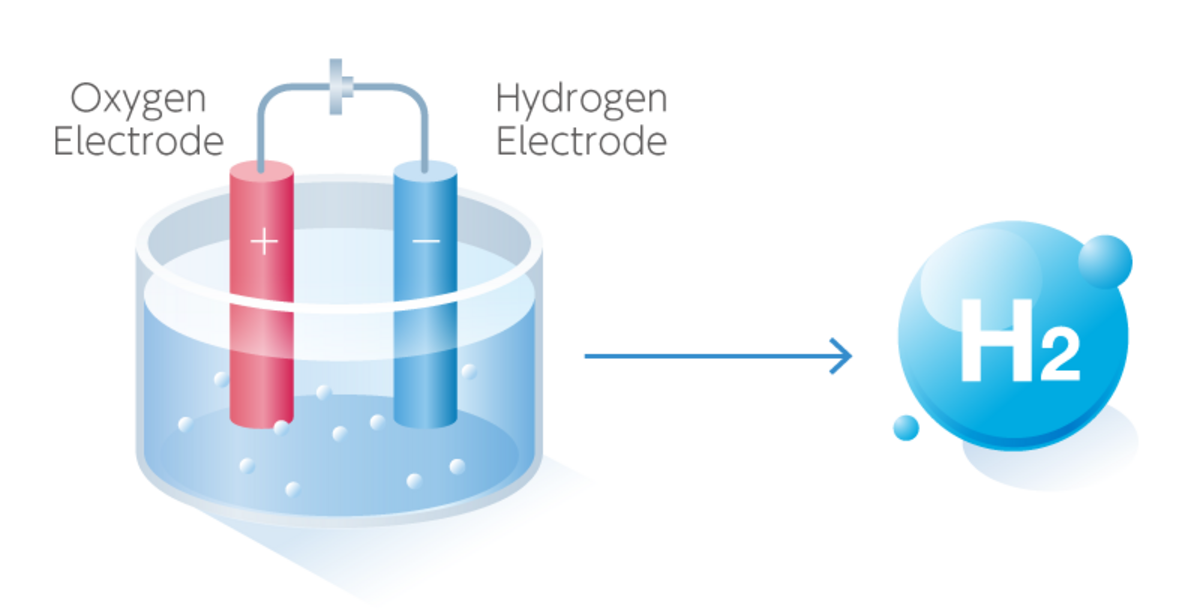
Water What Is Electrolysis . The ions move to the opposite. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: Ions move to opposite electrodes,. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes. It involves passing an electric current through the water, which results in the decomposition of water. However, because pure water is a very. H 2 and o 2.
from www.horiba.com
The ions move to the opposite. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes. The electrolysis of water produces hydrogen and oxygen gases. However, because pure water is a very. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: It involves passing an electric current through the water, which results in the decomposition of water. H 2 and o 2. Ions move to opposite electrodes,. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases.
Water Electrolysis Evaluation Hydrogen Energy HORIBA
Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The electrolytic cell consists of a pair of platinum electrodes. Ions move to opposite electrodes,. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. H 2 and o 2. The ions move to the opposite. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. However, because pure water is a very. It involves passing an electric current through the water, which results in the decomposition of water. The electrolysis of water produces hydrogen and oxygen gases.
From mungfali.com
Water Electrolysis Process Water What Is Electrolysis Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The electrolytic cell consists of a pair of platinum electrodes. H 2 and o 2. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. However, because pure water is a very. It involves passing an electric current through the. Water What Is Electrolysis.
From mungfali.com
Water Electrolysis Process Water What Is Electrolysis Ions move to opposite electrodes,. It involves passing an electric current through the water, which results in the decomposition of water. However, because pure water is a very. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is mainly carried out. Water What Is Electrolysis.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 8 Water What Is Electrolysis The ions move to the opposite. Ions move to opposite electrodes,. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: However, because pure water is a very. H 2 and o 2. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. It involves passing an electric current through. Water What Is Electrolysis.
From www.vecteezy.com
Electrolysis diagram experiment for education 1610466 Vector Art at Water What Is Electrolysis The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. It involves passing an electric current through the water, which results in the decomposition of water. H 2 and o 2. The electrolytic cell consists of a pair of platinum electrodes. However,. Water What Is Electrolysis.
From www.yaclass.in
Electrolysis of Water — lesson. Science CBSE, Class 10. Water What Is Electrolysis Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: However, because pure water is a very. The ions move to the opposite. Ions move to opposite electrodes,. The electrolytic cell consists of a pair of platinum electrodes. The electrolysis of water produces hydrogen and oxygen gases. It involves passing an electric current. Water What Is Electrolysis.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. H 2 and o 2. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent. Water What Is Electrolysis.
From electrolysisofwater.blogspot.com
Electrolysis of Water Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Ions move to opposite electrodes,. However, because pure water is a very. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The ions move to the opposite. Electrolysis can also. Water What Is Electrolysis.
From www.scienceprojectideas.org
Electrolysis of Water Experiment Science Project Ideas Water What Is Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ions move to opposite electrodes,. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. It involves passing an electric current through the water,. Water What Is Electrolysis.
From www.enagic-asia.com
The electrolysis process Enagic Kangen Water Water What Is Electrolysis The ions move to the opposite. It involves passing an electric current through the water, which results in the decomposition of water. H 2 and o 2. The electrolytic cell consists of a pair of platinum electrodes. However, because pure water is a very. Electrolysis, process by which electric current is passed through a substance to effect a chemical change.. Water What Is Electrolysis.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Water What Is Electrolysis The ions move to the opposite. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: Ions move to opposite electrodes,. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. However, because pure water is a very. The electrolytic cell consists of a pair of platinum electrodes. It involves. Water What Is Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Water What Is Electrolysis The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite. However, because pure water is a very. It involves passing an electric current through the water, which results in the decomposition of water. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a. Water What Is Electrolysis.
From www.researchgate.net
The fundamental of water electrolysis process. Download Scientific Water What Is Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The ions move to the opposite. It involves passing an electric current through the water, which results in the decomposition of water. H 2 and o 2. Electrolysis can also be. Water What Is Electrolysis.
From www.alamy.com
water electrolysis Cut Out Stock Images & Pictures Alamy Water What Is Electrolysis Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The electrolysis of water produces hydrogen and oxygen gases. H 2 and o 2. However, because pure water is a very. It involves passing an electric current through the water, which results in the decomposition of water. The ions move to the opposite.. Water What Is Electrolysis.
From vectormine.com
Water electrolysis process, scientific chemistry diagram VectorMine Water What Is Electrolysis Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. However, because pure water. Water What Is Electrolysis.
From www.dreamstime.com
Electrolysis of Water Forming Hydrogen and Oxygen Vector Illustration Water What Is Electrolysis It involves passing an electric current through the water, which results in the decomposition of water. The ions move to the opposite. The electrolytic cell consists of a pair of platinum electrodes. However, because pure water is a very. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Electrolysis, process by which electric current is passed. Water What Is Electrolysis.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Water What Is Electrolysis Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. It involves passing an electric. Water What Is Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Water What Is Electrolysis Ions move to opposite electrodes,. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. However, because pure water is a very. It involves passing an electric current through the water, which results in the decomposition of water. H 2 and. Water What Is Electrolysis.
From www.alamy.com
Water electrolysis hires stock photography and images Alamy Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. It involves passing an electric current through the water, which results in the decomposition of water. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is. Water What Is Electrolysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Water What Is Electrolysis Ions move to opposite electrodes,. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. It involves passing an electric current through the water, which results in the decomposition of water. Electrolysis, process by which electric current is passed through a substance to effect a. Water What Is Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Water What Is Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolysis of water produces hydrogen and oxygen gases. It involves passing an electric current through the water, which results in the decomposition of water. However, because pure water is a. Water What Is Electrolysis.
From www.hotelsrate.org
Water Electrolysis Equation Modern Home Designs Water What Is Electrolysis Ions move to opposite electrodes,. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite. It involves passing an electric current through the water, which results in the decomposition of water. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The electrolytic cell consists of a pair. Water What Is Electrolysis.
From www.horiba.com
Water Electrolysis Evaluation Hydrogen Energy HORIBA Water What Is Electrolysis However, because pure water is a very. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Ions move to opposite electrodes,. H 2 and o 2. The ions move to the opposite. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. Water electrolysis. Water What Is Electrolysis.
From www.researchgate.net
Schematic of water electrolysis system. Download Scientific Diagram Water What Is Electrolysis It involves passing an electric current through the water, which results in the decomposition of water. However, because pure water is a very. The electrolytic cell consists of a pair of platinum electrodes. H 2 and o 2. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: The ions move to the. Water What Is Electrolysis.
From www.alamy.com
Electrolysis of water hires stock photography and images Alamy Water What Is Electrolysis The electrolysis of water produces hydrogen and oxygen gases. H 2 and o 2. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. However, because pure water is a very. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: It involves passing an electric current. Water What Is Electrolysis.
From www.alamy.com
Electrolysis of water, illustration Stock Vector Images Alamy Water What Is Electrolysis Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. However, because pure water is a very. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: Electrolysis, process by which electric current is passed through a substance to effect. Water What Is Electrolysis.
From theedge.com.hk
waterelectrolysis The Edge Water What Is Electrolysis H 2 and o 2. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes. Ions move to opposite electrodes,. The electrolysis of water produces hydrogen and oxygen gases. However, because. Water What Is Electrolysis.
From quizlet.com
Electrolysis of Water Experiment Explanation Diagram Quizlet Water What Is Electrolysis H 2 and o 2. It involves passing an electric current through the water, which results in the decomposition of water. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: However, because pure water is a very. Electrolysis, process by which electric current is passed through a substance to effect a chemical. Water What Is Electrolysis.
From www.jargonium.com
Electrolysis and Education Learning Models not Facts Water What Is Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. It involves passing an electric current through the water, which results in the decomposition of water. Ions move to opposite electrodes,. The electrolysis of water produces hydrogen and oxygen gases. However, because pure water is a very. H 2 and o 2. Electrolysis can. Water What Is Electrolysis.
From www.sciencephoto.com
Electrolysis of water, illustration Stock Image C050/8178 Science Water What Is Electrolysis Ions move to opposite electrodes,. The electrolytic cell consists of a pair of platinum electrodes. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. H 2 and o 2. It involves passing an electric current through the water, which results in the decomposition of. Water What Is Electrolysis.
From www.vectorstock.com
Water electrolysis diagram Royalty Free Vector Image Water What Is Electrolysis However, because pure water is a very. Ions move to opposite electrodes,. The ions move to the opposite. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: It involves. Water What Is Electrolysis.
From www.h2bulletin.com
Hydrogen production through electrolysis H2 Bulletin Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Ions move to opposite electrodes,. The ions move to the opposite. However, because pure water is a very. It involves passing an electric current through the water, which results in the decomposition of water. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolysis of. Water What Is Electrolysis.
From www.wikihow.com
How to Electrolyse Water 12 Steps (with Pictures) wikiHow Water What Is Electrolysis Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolysis of water produces hydrogen and oxygen gases. It involves passing an electric current through the water, which results in the decomposition of water. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. H 2 and o 2. The. Water What Is Electrolysis.
From electrolysisofwater.blogspot.com
Electrolysis of Water Water What Is Electrolysis The electrolytic cell consists of a pair of platinum electrodes. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. It involves passing an electric current through the water, which results in the decomposition of water. The electrolysis of water produces. Water What Is Electrolysis.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Water What Is Electrolysis H 2 and o 2. Water electrolysis is mainly carried out to yield pure hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to drive the thermodynamically nonspontaneous decomposition of water into its constituent elements: However, because pure water is a very. Ions move to opposite electrodes,. Electrolysis, process by. Water What Is Electrolysis.
From books.studentsource.org
Electrolysis Water What Is Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. However, because pure water is a very. H 2 and o 2. It involves passing an electric current through the water, which results in the decomposition of water. The ions move to the opposite. The electrolytic cell consists of a pair of platinum electrodes.. Water What Is Electrolysis.