Ph And Buffers Lab . A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. accurately measure the ph of solutions using ph indicator strips and a ph meter. Skip to main content if you're seeing this message, it means we're having trouble loading. Create buffer solutions and test the. Students will determine the ph of various common. decide on ph for experiment, then choose buffer with pka close to ph. this lab introduces students to ph, ph indicators, and buffers. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. acidity and basicity, proton concentration, the ph scale, and buffers. ph is the measure of acidity or basicity of a solution. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Pka of buffer should be within 1 ph unit of solution ph. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or.
from www.transtutors.com
decide on ph for experiment, then choose buffer with pka close to ph. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Students will determine the ph of various common. ph is the measure of acidity or basicity of a solution. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. Pka of buffer should be within 1 ph unit of solution ph. accurately measure the ph of solutions using ph indicator strips and a ph meter. this lab introduces students to ph, ph indicators, and buffers. acidity and basicity, proton concentration, the ph scale, and buffers.
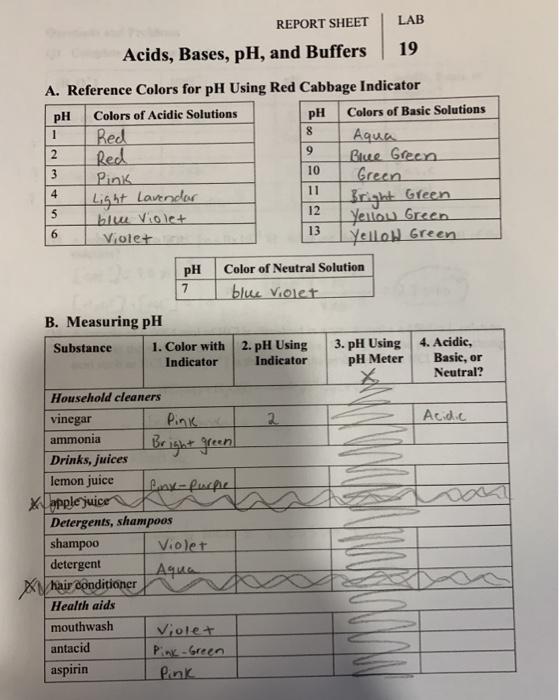
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A
Ph And Buffers Lab Students will determine the ph of various common. Students will determine the ph of various common. ph is the measure of acidity or basicity of a solution. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. decide on ph for experiment, then choose buffer with pka close to ph. acidity and basicity, proton concentration, the ph scale, and buffers. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. Pka of buffer should be within 1 ph unit of solution ph. Create buffer solutions and test the. this lab introduces students to ph, ph indicators, and buffers. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Skip to main content if you're seeing this message, it means we're having trouble loading. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. accurately measure the ph of solutions using ph indicator strips and a ph meter.
From www.youtube.com
pH Measurements—Buffers and Their Properties Lab YouTube Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. this lab introduces students to ph, ph indicators, and buffers.. Ph And Buffers Lab.
From www.youtube.com
Lab 8 Acids, Bases, and Buffers experiment YouTube Ph And Buffers Lab Students will determine the ph of various common. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. accurately measure the ph of solutions using ph indicator strips and a ph meter. A ph value is a number, usually between 0 and 14, that represents the acidity,. Ph And Buffers Lab.
From www.youtube.com
EXPERIMENT Preparation of buffer solution and measurement of pH Ph And Buffers Lab accurately measure the ph of solutions using ph indicator strips and a ph meter. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. Create buffer solutions and test the. Tightly maintaining the ph to the desired range is required in. Ph And Buffers Lab.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Ph And Buffers Lab ph is the measure of acidity or basicity of a solution. acidity and basicity, proton concentration, the ph scale, and buffers. Skip to main content if you're seeing this message, it means we're having trouble loading. Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between 0 and. Ph And Buffers Lab.
From www.scribd.com
pH and Buffers Acid Buffer Solution Ph And Buffers Lab Create buffer solutions and test the. Students will determine the ph of various common. Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. rather than simply measuring the ph of a solution, you may wish. Ph And Buffers Lab.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. ph is the measure of acidity or basicity of a solution. Students will determine the ph of various common. Create buffer solutions and test the. accurately measure the ph of solutions using ph indicator strips and a ph meter. Pka of buffer should be within 1 ph. Ph And Buffers Lab.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Ph And Buffers Lab ph is the measure of acidity or basicity of a solution. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Create buffer solutions and test the. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure. Ph And Buffers Lab.
From www.coleparmer.com
Thermo Scientific Orion Standard All in One pH Buffer Kit from ColeParmer Ph And Buffers Lab Pka of buffer should be within 1 ph unit of solution ph. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. Skip to main content if you're seeing this message, it. Ph And Buffers Lab.
From www.scribd.com
PHA6112 Lab PH and BuffersDraft PDF Ph Buffer Solution Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. ph is the measure of acidity or basicity of a solution. Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. to understand how. Ph And Buffers Lab.
From www.chegg.com
Acids, Bases, Buffers and pH Postlaboratory Ph And Buffers Lab Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. acidity and basicity, proton concentration, the ph scale, and buffers. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. Skip to main content. Ph And Buffers Lab.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Ph And Buffers Lab this lab introduces students to ph, ph indicators, and buffers. Students will determine the ph of various common. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. Skip to main content if you're seeing this message, it means we're having. Ph And Buffers Lab.
From www.slideserve.com
PPT Lab Activity 2 Active Acidity, pH, and Buffer PowerPoint Ph And Buffers Lab this lab introduces students to ph, ph indicators, and buffers. decide on ph for experiment, then choose buffer with pka close to ph. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. accurately measure the ph of solutions using ph indicator strips and a ph. Ph And Buffers Lab.
From fyozskydn.blob.core.windows.net
Ph And Buffers Lab Answers at Eric Swain blog Ph And Buffers Lab Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. Skip to main content if. Ph And Buffers Lab.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Ph And Buffers Lab accurately measure the ph of solutions using ph indicator strips and a ph meter. Students will determine the ph of various common. ph is the measure of acidity or basicity of a solution. Create buffer solutions and test the. Skip to main content if you're seeing this message, it means we're having trouble loading. rather than simply. Ph And Buffers Lab.
From exydsrovs.blob.core.windows.net
How To Use Buffer Solution Ph at Clint Stacey blog Ph And Buffers Lab Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. accurately measure the ph of solutions using ph indicator strips and a ph meter. Students will determine the ph of various common. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation. Ph And Buffers Lab.
From www.mcguffmedical.com
pH Calibrating Buffer Solution, pH 4.01, 475mL, Each McGuff Medical Ph And Buffers Lab Students will determine the ph of various common. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. accurately measure the ph of solutions using ph indicator strips and a ph meter. Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between. Ph And Buffers Lab.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Ph And Buffers Lab decide on ph for experiment, then choose buffer with pka close to ph. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. ph is the measure of acidity or basicity of a solution. Skip to main content if you're seeing this message, it means we're having. Ph And Buffers Lab.
From www.youtube.com
pH and Buffers Lab YouTube Ph And Buffers Lab this lab introduces students to ph, ph indicators, and buffers. acidity and basicity, proton concentration, the ph scale, and buffers. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. to understand how well a buffer protects against changes in ph, consider the effect of. Ph And Buffers Lab.
From www.ubuy.co.in
Buy Bante 210 Benchtop pH Meter Lab pH Meter for Routine Measurements Ph And Buffers Lab accurately measure the ph of solutions using ph indicator strips and a ph meter. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. Create buffer solutions and test the. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or. Ph And Buffers Lab.
From fyozskydn.blob.core.windows.net
Ph And Buffers Lab Answers at Eric Swain blog Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. Pka of buffer should be within 1 ph unit of solution ph. Students will determine the ph of various common. A. Ph And Buffers Lab.
From www.numerade.com
SOLVED REPORT SHEET LAB Acids, Bases, pH, and Buffers Reference Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. Pka of buffer should be within 1 ph unit of solution ph. Create buffer solutions and test the. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. to understand how well a buffer protects against. Ph And Buffers Lab.
From www.youtube.com
Buffers and pH Biology YouTube Ph And Buffers Lab acidity and basicity, proton concentration, the ph scale, and buffers. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Pka of buffer should. Ph And Buffers Lab.
From www.chegg.com
Solved Acids Bases, pH, and Buffers Lab Information 21/ hr Ph And Buffers Lab ph is the measure of acidity or basicity of a solution. this lab introduces students to ph, ph indicators, and buffers. Pka of buffer should be within 1 ph unit of solution ph. Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. A ph value is a number, usually between. Ph And Buffers Lab.
From www.fishersci.fi
Buffer Solution, pH 7.00, ChemLab Buffers Buffers and Solutions Ph And Buffers Lab Students will determine the ph of various common. Create buffer solutions and test the. acidity and basicity, proton concentration, the ph scale, and buffers. Skip to main content if you're seeing this message, it means we're having trouble loading. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation. Ph And Buffers Lab.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Ph And Buffers Lab to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. acidity and basicity, proton concentration, the ph scale, and buffers. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution.. Ph And Buffers Lab.
From www.studocu.com
Lab 05 p H and Buffers Lab report. Lab pH and Buffers Ph And Buffers Lab rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. Create buffer solutions and test the. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. acidity and basicity,. Ph And Buffers Lab.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Ph And Buffers Lab Skip to main content if you're seeing this message, it means we're having trouble loading. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or. Ph And Buffers Lab.
From www.chegg.com
Solved Lab 3 pH and Buffers Discussion A. The pH scale and Ph And Buffers Lab Tightly maintaining the ph to the desired range is required in all experiments, and buffer solutions help. Pka of buffer should be within 1 ph unit of solution ph. Skip to main content if you're seeing this message, it means we're having trouble loading. Students will determine the ph of various common. rather than simply measuring the ph of. Ph And Buffers Lab.
From www.youtube.com
Lab 3 Understanding pH and Buffers Video Narration YouTube Ph And Buffers Lab Students will determine the ph of various common. Skip to main content if you're seeing this message, it means we're having trouble loading. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. to understand how well a buffer protects against changes in ph, consider the effect of. Ph And Buffers Lab.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Ph And Buffers Lab Create buffer solutions and test the. Students will determine the ph of various common. acidity and basicity, proton concentration, the ph scale, and buffers. accurately measure the ph of solutions using ph indicator strips and a ph meter. ph is the measure of acidity or basicity of a solution. to understand how well a buffer protects. Ph And Buffers Lab.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Ph And Buffers Lab Students will determine the ph of various common. Pka of buffer should be within 1 ph unit of solution ph. accurately measure the ph of solutions using ph indicator strips and a ph meter. this lab introduces students to ph, ph indicators, and buffers. Skip to main content if you're seeing this message, it means we're having trouble. Ph And Buffers Lab.
From www.youtube.com
pH and Buffers Lab Instructions YouTube Ph And Buffers Lab this lab introduces students to ph, ph indicators, and buffers. Skip to main content if you're seeing this message, it means we're having trouble loading. Students will determine the ph of various common. Pka of buffer should be within 1 ph unit of solution ph. A ph value is a number, usually between 0 and 14, that represents the. Ph And Buffers Lab.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Ph And Buffers Lab to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume. rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. ph is the measure of acidity or basicity of. Ph And Buffers Lab.
From fyojkjzvi.blob.core.windows.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Lab Report at Martin Ph And Buffers Lab rather than simply measuring the ph of a solution, you may wish to control the ph, during edta complexation titrations or. acidity and basicity, proton concentration, the ph scale, and buffers. A ph value is a number, usually between 0 and 14, that represents the acidity, neutrality or basicity of a solution. Create buffer solutions and test the.. Ph And Buffers Lab.
From www.studocu.com
Acids, Bases, and p H Buffers SA answers Drylab Acids, Bases, and Ph And Buffers Lab accurately measure the ph of solutions using ph indicator strips and a ph meter. decide on ph for experiment, then choose buffer with pka close to ph. Create buffer solutions and test the. ph is the measure of acidity or basicity of a solution. Pka of buffer should be within 1 ph unit of solution ph. A. Ph And Buffers Lab.