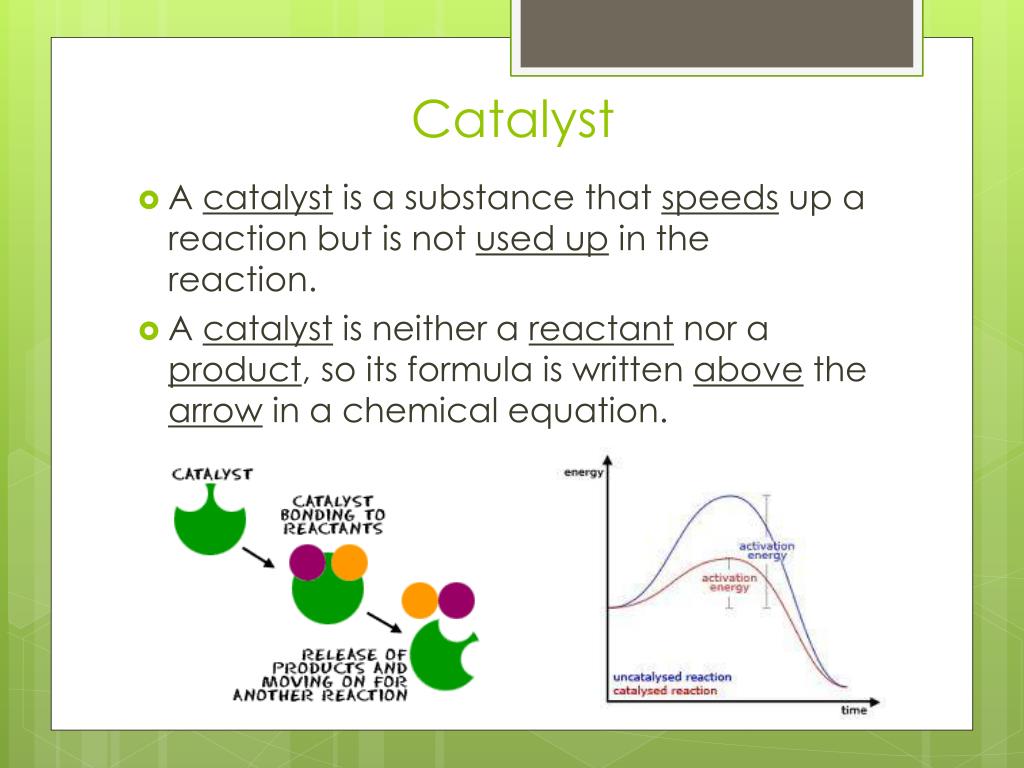
How Do Catalysts Affect A Chemical Reaction . The catalyst is not used up or chemically changed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This process is called catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Catalysts participate in a chemical reaction and increase its rate. See examples, diagrams and explanations of catalysis and collision theory. Adsorption and intermediate compounds, with examples and activities. Explore two types of catalysts: Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. List examples of catalysis in natural. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst is a substance that speeds up a chemical reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.
from www.slideserve.com
A catalyst is a substance that speeds up a chemical reaction. See examples, diagrams and explanations of catalysis and collision theory. Catalysts participate in a chemical reaction and increase its rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. List examples of catalysis in natural. Adsorption and intermediate compounds, with examples and activities. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explore two types of catalysts:
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
How Do Catalysts Affect A Chemical Reaction A catalyst is a substance that speeds up a chemical reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The catalyst is not used up or chemically changed during the reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts participate in a chemical reaction and increase its rate. Explore two types of catalysts: See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. This process is called catalysis. See examples, diagrams and explanations of catalysis and collision theory. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. Adsorption and intermediate compounds, with examples and activities.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation. How Do Catalysts Affect A Chemical Reaction.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Adsorption and intermediate compounds, with examples and activities. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. How Do Catalysts Affect A Chemical Reaction.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube How Do Catalysts Affect A Chemical Reaction A catalyst is a substance that speeds up a chemical reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. This process is called catalysis. Explain the function of a catalyst in terms of reaction mechanisms and. How Do Catalysts Affect A Chemical Reaction.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. List examples of catalysis in natural. This process is called catalysis. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. See examples of catalyzed reactions. How Do Catalysts Affect A Chemical Reaction.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst How Do Catalysts Affect A Chemical Reaction List examples of catalysis in natural. This process is called catalysis. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. The catalyst is not used up or chemically changed during the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction. How Do Catalysts Affect A Chemical Reaction.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free How Do Catalysts Affect A Chemical Reaction See examples, diagrams and explanations of catalysis and collision theory. Adsorption and intermediate compounds, with examples and activities. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. The catalyst is not used up or chemically changed during the reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential. How Do Catalysts Affect A Chemical Reaction.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation How Do Catalysts Affect A Chemical Reaction Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. List examples of catalysis in natural. Adsorption and intermediate compounds, with examples and activities. They do not appear in the reaction’s net. How Do Catalysts Affect A Chemical Reaction.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry How Do Catalysts Affect A Chemical Reaction Catalysts participate in a chemical reaction and increase its rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a chemical substance that affects the rate of a chemical. How Do Catalysts Affect A Chemical Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Adsorption and intermediate compounds, with examples and activities. See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. A catalyst is a chemical substance that affects. How Do Catalysts Affect A Chemical Reaction.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis How Do Catalysts Affect A Chemical Reaction Adsorption and intermediate compounds, with examples and activities. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. See examples, diagrams and explanations of catalysis and collision theory. This process is called catalysis. Explore two types of catalysts: Learn. How Do Catalysts Affect A Chemical Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Do Catalysts Affect A Chemical Reaction Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts participate in a chemical reaction and increase its rate. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. List examples of catalysis in natural. Explore two types of catalysts: Explain the function of a catalyst in terms of. How Do Catalysts Affect A Chemical Reaction.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of How Do Catalysts Affect A Chemical Reaction Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. The catalyst is not used up or chemically changed during the reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams;. How Do Catalysts Affect A Chemical Reaction.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile How Do Catalysts Affect A Chemical Reaction List examples of catalysis in natural. This process is called catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. Explore two types of catalysts: Adsorption and intermediate compounds, with examples and activities. See examples, diagrams. How Do Catalysts Affect A Chemical Reaction.
From www.youtube.com
Identifying catalysts in a reaction YouTube How Do Catalysts Affect A Chemical Reaction See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. The catalyst is not used up or chemically changed during the reaction. This process is called catalysis. Catalysts participate in a chemical reaction and increase its rate.. How Do Catalysts Affect A Chemical Reaction.
From 2012books.lardbucket.org
Catalysis How Do Catalysts Affect A Chemical Reaction Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. The catalyst is not used up or chemically changed during the reaction. They do not appear in the reaction’s net equation and are not consumed. How Do Catalysts Affect A Chemical Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 How Do Catalysts Affect A Chemical Reaction Explore two types of catalysts: See examples, diagrams and explanations of catalysis and collision theory. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. They do. How Do Catalysts Affect A Chemical Reaction.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation How Do Catalysts Affect A Chemical Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This process is called catalysis. The catalyst is not used up or chemically changed during the reaction. Explore two types of catalysts: A catalyst is a substance that speeds up a chemical reaction. Learn how catalysts speed up reactions by providing. How Do Catalysts Affect A Chemical Reaction.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst How Do Catalysts Affect A Chemical Reaction A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Adsorption and intermediate compounds, with examples and activities. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Learn how catalysts speed up reactions by providing. How Do Catalysts Affect A Chemical Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Do Catalysts Affect A Chemical Reaction Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. They do not appear in the reaction’s net equation and are not consumed during the reaction. Adsorption and intermediate compounds, with examples and activities. List examples of catalysis in natural. A catalyst is not consumed by the reaction and it may participate in. How Do Catalysts Affect A Chemical Reaction.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions How Do Catalysts Affect A Chemical Reaction A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts participate in a chemical reaction and increase its rate. The catalyst is not used up or chemically changed during the reaction. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. Explore two types of catalysts:. How Do Catalysts Affect A Chemical Reaction.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog How Do Catalysts Affect A Chemical Reaction Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts participate in a chemical reaction and increase its rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. The catalyst. How Do Catalysts Affect A Chemical Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Do Catalysts Affect A Chemical Reaction Explore two types of catalysts: The catalyst is not used up or chemically changed during the reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. Explain the function of a catalyst in terms of reaction. How Do Catalysts Affect A Chemical Reaction.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint How Do Catalysts Affect A Chemical Reaction This process is called catalysis. The catalyst is not used up or chemically changed during the reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. Catalysts allow a reaction to proceed via a. How Do Catalysts Affect A Chemical Reaction.
From www.thoughtco.com
Catalysis Definition in Chemistry How Do Catalysts Affect A Chemical Reaction List examples of catalysis in natural. Catalysts participate in a chemical reaction and increase its rate. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. Adsorption and intermediate compounds, with examples and activities. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Explore. How Do Catalysts Affect A Chemical Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. List examples of catalysis in natural. A catalyst is a substance that speeds up a chemical reaction. This process is called catalysis. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is not consumed by the reaction and it may. How Do Catalysts Affect A Chemical Reaction.
From www.nagwa.com
Question Video Determining How Catalysts Affect the Energy Changes How Do Catalysts Affect A Chemical Reaction The catalyst is not used up or chemically changed during the reaction. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. Catalysts participate in a chemical reaction and increase its rate. See examples, diagrams and explanations of catalysis and collision theory. Learn how catalysts affect the rate of chemical reactions by changing the likelihood. How Do Catalysts Affect A Chemical Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Do Catalysts Affect A Chemical Reaction See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Explore two types of catalysts: A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation. How Do Catalysts Affect A Chemical Reaction.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B How Do Catalysts Affect A Chemical Reaction See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. They do not appear in the reaction’s net equation and are not consumed during the reaction. Explore two types of catalysts: Catalysts allow a reaction to proceed. How Do Catalysts Affect A Chemical Reaction.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent How Do Catalysts Affect A Chemical Reaction Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up a chemical reaction. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. See examples of catalyzed reactions and their. How Do Catalysts Affect A Chemical Reaction.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Do Catalysts Affect A Chemical Reaction Learn how catalysts affect the rate of chemical reactions by changing the likelihood of collisions between particles. This process is called catalysis. List examples of catalysis in natural. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential. How Do Catalysts Affect A Chemical Reaction.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free How Do Catalysts Affect A Chemical Reaction Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in natural. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower. How Do Catalysts Affect A Chemical Reaction.
From www.slideshare.net
Biology 2.4 How Do Catalysts Affect A Chemical Reaction Adsorption and intermediate compounds, with examples and activities. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. See examples, diagrams and explanations of catalysis and collision theory. Catalysts participate in a chemical reaction and. How Do Catalysts Affect A Chemical Reaction.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction How Do Catalysts Affect A Chemical Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. A catalyst is a substance that speeds up a chemical reaction. This process is called catalysis. A catalyst is a chemical substance that affects the rate. How Do Catalysts Affect A Chemical Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Do Catalysts Affect A Chemical Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. See examples of catalyzed reactions and their catalysts, and how they affect the rate of reaction. Learn how catalysts affect the rate of chemical reactions. How Do Catalysts Affect A Chemical Reaction.
From courses.lumenlearning.com
Catalysis Chemistry for Majors How Do Catalysts Affect A Chemical Reaction Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn how catalysts speed up reactions by providing an alternative pathway with lower activation energy. They do not appear in the reaction’s net equation and are not. How Do Catalysts Affect A Chemical Reaction.