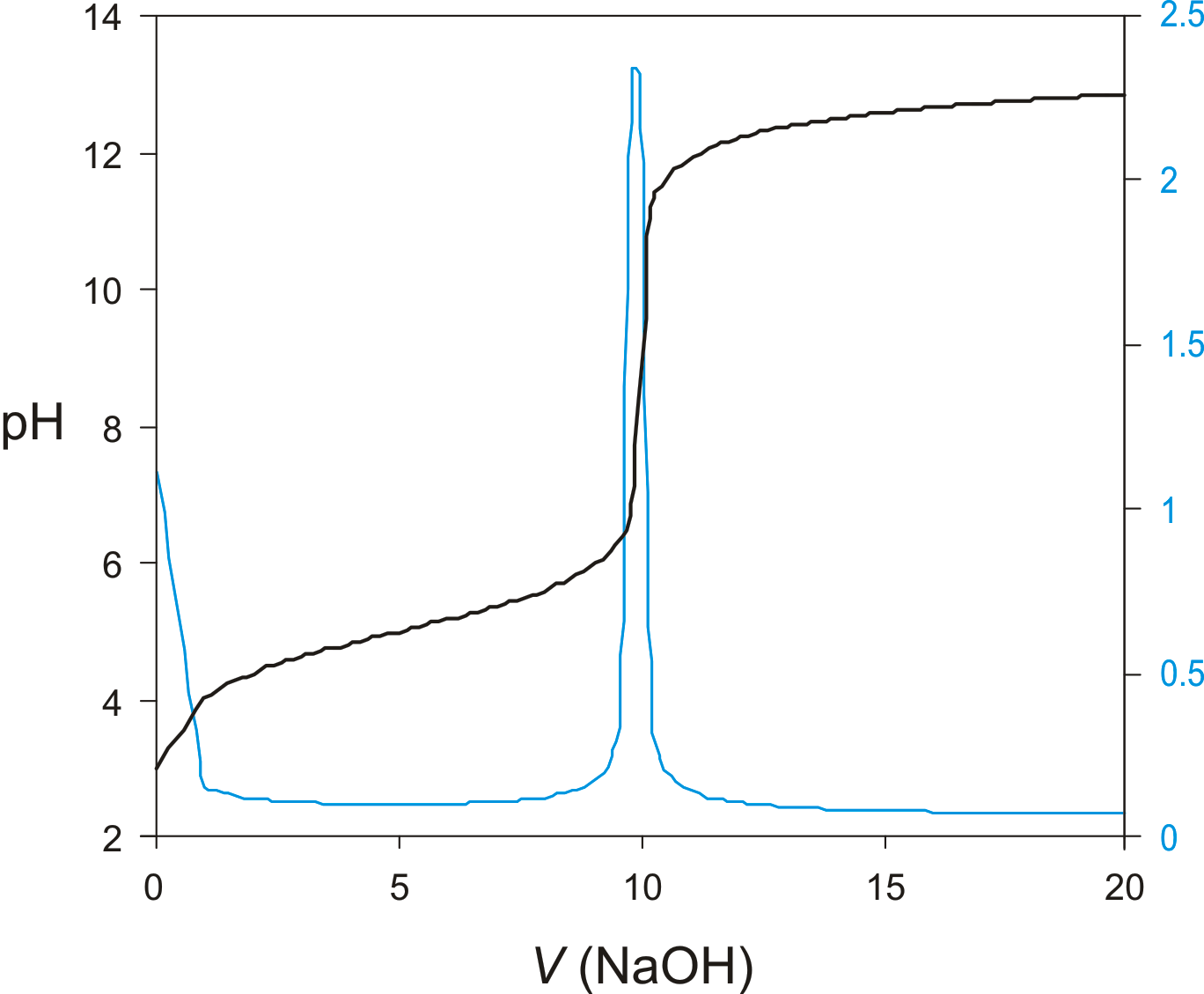
Titration Curve Definition . how do you explain the shape of a titration curve? The figure below shows two different. Everything you need to know for a level The titration curve is a graphical representation of the ph or other property changes during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. And why is the equivalence point not always at ph7? a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong acid.
from glossary.periodni.com
how do you explain the shape of a titration curve? The figure below shows two different examples of a strong acid. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? The figure below shows two different. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration. The end point of a titration is the point at.
Titration curve Chemistry Dictionary & Glossary
Titration Curve Definition a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Everything you need to know for a level a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. The figure below shows two different. The figure below shows two different examples of a strong acid. how do you explain the shape of a titration curve? The titration curve is a graphical representation of the ph or other property changes during a titration. And why is the equivalence point not always at ph7? a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Definition Everything you need to know for a level The figure below shows two different examples of a strong acid. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration. Titration Curve Definition.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Definition The figure below shows two different examples of a strong acid. The titration curve is a graphical representation of the ph or other property changes during a titration. The end point of a titration is the point at. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows. Titration Curve Definition.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Definition how do you explain the shape of a titration curve? The figure below shows two different examples of a strong acid. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Everything you need to know for a level The end point of a titration. Titration Curve Definition.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Definition The end point of a titration is the point at. how do you explain the shape of a titration curve? titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. And why is the equivalence point not always at ph7? a titration curve is. Titration Curve Definition.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Titration Curve Definition The figure below shows two different examples of a strong acid. And why is the equivalence point not always at ph7? The figure below shows two different. Everything you need to know for a level titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. . Titration Curve Definition.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID89337 Titration Curve Definition The figure below shows two different. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. And why is the equivalence point not always at ph7? The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a. Titration Curve Definition.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration Curve Definition a titration curve is a graphical representation of the ph of a solution during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration. The figure below shows two different examples of. Titration Curve Definition.
From exyrafpmt.blob.core.windows.net
Titration Curve Using at Nancy Madsen blog Titration Curve Definition The end point of a titration is the point at. how do you explain the shape of a titration curve? a titration curve is a graphical representation of the ph of a solution during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. And why is the. Titration Curve Definition.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Definition The titration curve is a graphical representation of the ph or other property changes during a titration. And why is the equivalence point not always at ph7? The end point of a titration is the point at. The figure below shows two different. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium. Titration Curve Definition.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Curve Definition Everything you need to know for a level a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration. The. Titration Curve Definition.
From giotsjinw.blob.core.windows.net
Titration Definition In Short at Angel Serrano blog Titration Curve Definition how do you explain the shape of a titration curve? titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during. Titration Curve Definition.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Titration Curve Definition And why is the equivalence point not always at ph7? how do you explain the shape of a titration curve? a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. a titration curve is a graphical representation of the ph of. Titration Curve Definition.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Definition The figure below shows two different examples of a strong acid. how do you explain the shape of a titration curve? a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is. Titration Curve Definition.
From pressbooks.bccampus.ca
14.7 AcidBase Titrations Chemistry 2e for Chem 120 (Chemistry for Titration Curve Definition The titration curve is a graphical representation of the ph or other property changes during a titration. Everything you need to know for a level how do you explain the shape of a titration curve? The figure below shows two different examples of a strong acid. a titration curve is a graphical representation of the ph of a. Titration Curve Definition.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Definition Everything you need to know for a level a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration. The end point of a titration is the point at. a titration curve is a graphical. Titration Curve Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve Definition The figure below shows two different. And why is the equivalence point not always at ph7? The titration curve is a graphical representation of the ph or other property changes during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The end point of a titration is the point. Titration Curve Definition.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Definition The end point of a titration is the point at. Everything you need to know for a level a titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration. titration curves show how the ph of. Titration Curve Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Titration Curve Definition how do you explain the shape of a titration curve? a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong acid. The end point. Titration Curve Definition.
From quizlet.com
Weak Acid Strong Base Titration Curve Particle View Diagram Quizlet Titration Curve Definition titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The end point of a titration is the point at. The titration. Titration Curve Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Titration Curve Definition a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? The end point of a titration is the point at. how do you explain. Titration Curve Definition.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Definition The titration curve is a graphical representation of the ph or other property changes during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation. Titration Curve Definition.
From www.jove.com
AcidBase Titration Curves JoVE Titration Curve Definition Everything you need to know for a level The figure below shows two different. And why is the equivalence point not always at ph7? titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at. The figure. Titration Curve Definition.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Definition titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. And why is the equivalence point not always at. Titration Curve Definition.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5570905 Titration Curve Definition a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong acid. Everything you need to know for a level And why is the equivalence point not always at ph7? a titration curve is a graphical representation of the ph of a. Titration Curve Definition.
From mavink.com
Buffer Region Titration Curve Titration Curve Definition And why is the equivalence point not always at ph7? Everything you need to know for a level a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The end point of a titration is the point at. The titration curve is a graphical representation of the ph or other property changes. Titration Curve Definition.
From glossary.periodni.com
Titration curve Chemistry Dictionary & Glossary Titration Curve Definition The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong acid. titration curves show. Titration Curve Definition.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Definition The titration curve is a graphical representation of the ph or other property changes during a titration. Everything you need to know for a level The end point of a titration is the point at. The figure below shows two different examples of a strong acid. a titration curve is a graphical representation of the ph of a solution. Titration Curve Definition.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Definition And why is the equivalence point not always at ph7? The end point of a titration is the point at. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. how do you explain the shape of a titration curve? The figure below shows two different. titration curves show how. Titration Curve Definition.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Definition The end point of a titration is the point at. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. how do you explain the shape of a titration curve? Everything you need to know for a level The figure below shows two different. . Titration Curve Definition.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Definition a titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a level The titration curve is a graphical representation of the ph or other property changes during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. And. Titration Curve Definition.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Curve Definition The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. titration curves show how the ph of an acidic or basic solution changes as. Titration Curve Definition.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Curve Definition how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? a typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration. a titration curve. Titration Curve Definition.
From generalchemistrylab.blogspot.co.uk
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Definition how do you explain the shape of a titration curve? a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. And why is the equivalence point not always. Titration Curve Definition.
From giohqmnus.blob.core.windows.net
Titration Definition Wikipedia at James Henrich blog Titration Curve Definition a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong acid. The titration curve is a graphical representation of the ph or other property changes during a titration. Everything you need to know for a level a typical titration curve of a. Titration Curve Definition.
From facts.net
10 Mindblowing Facts About Titration Curve Titration Curve Definition The end point of a titration is the point at. And why is the equivalence point not always at ph7? The titration curve is a graphical representation of the ph or other property changes during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a. Titration Curve Definition.