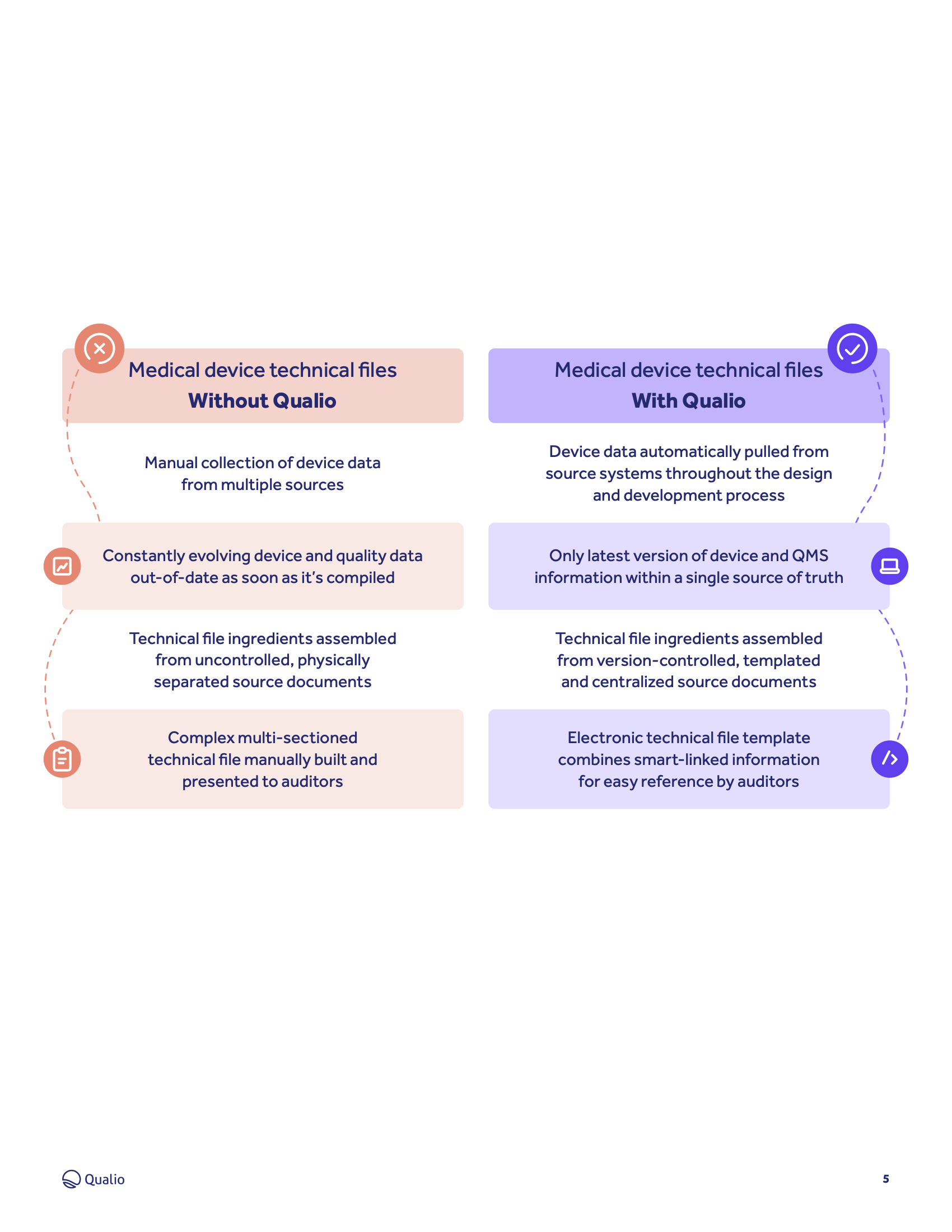
Technical File Medical Device Fda . Overview of regulations for medical devices: How should your file be structured and what software will you need? Iso 13485 requires a medical device file for each medical device type or medical device family. What are the medical device technical file requirements in iso 13485 and the eu mdr? Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Premarket notifications (510(k)), establishment registration, device listing, quality. Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Many manufacturers think that the medical device file is the.
from www.qualio.com
Iso 13485 requires a medical device file for each medical device type or medical device family. Premarket notifications (510(k)), establishment registration, device listing, quality. What are the medical device technical file requirements in iso 13485 and the eu mdr? Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. How should your file be structured and what software will you need? Many manufacturers think that the medical device file is the. Overview of regulations for medical devices: The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing.
Using Qualio for building a medical device technical file
Technical File Medical Device Fda Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Many manufacturers think that the medical device file is the. How should your file be structured and what software will you need? Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Overview of regulations for medical devices: What are the medical device technical file requirements in iso 13485 and the eu mdr? Premarket notifications (510(k)), establishment registration, device listing, quality. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Iso 13485 requires a medical device file for each medical device type or medical device family.
From www.qualio.com
Using Qualio for building a medical device technical file Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. What are the medical device technical file requirements in iso 13485 and the eu mdr? Your technical file must. Technical File Medical Device Fda.
From patientguard.com
How to structure a Medical Device Technical File Technical File Medical Device Fda Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Iso 13485 requires a medical device file for each medical device type or medical device family. How should your file be structured and what software will you need? What are the medical device technical file. Technical File Medical Device Fda.
From www.youtube.com
Design History File DHF, Device Master Record DMR, Device History Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Many. Technical File Medical Device Fda.
From www.tga.gov.au
Application audit (technical file review) of IVD medical device Technical File Medical Device Fda Many manufacturers think that the medical device file is the. Premarket notifications (510(k)), establishment registration, device listing, quality. What are the medical device technical file requirements in iso 13485 and the eu mdr? Overview of regulations for medical devices: Iso 13485 requires a medical device file for each medical device type or medical device family. Master files are created to. Technical File Medical Device Fda.
From www.greenlight.guru
Technical File vs. 510(k) vs. Design History File What Medical Device Technical File Medical Device Fda Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. What are the medical device technical file requirements in iso 13485 and the eu mdr? Overview of. Technical File Medical Device Fda.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Technical File Medical Device Fda Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Overview of regulations for medical devices: Premarket notifications (510(k)), establishment registration, device listing, quality. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Fda has developed this guidance. Technical File Medical Device Fda.
From www.slideteam.net
Five Years Medical Device Planning Roadmap With FDA Regulatory Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. Overview of regulations for medical devices: What are the medical device technical file requirements in iso 13485 and the eu mdr? The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Premarket notifications (510(k)), establishment registration,. Technical File Medical Device Fda.
From www.pinterest.com
QualiMEDtech is an authorized representative in USA. Contact us now for Technical File Medical Device Fda Overview of regulations for medical devices: Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Iso 13485 requires a medical device file for each medical device type or medical device family. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Master files are created. Technical File Medical Device Fda.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. Many manufacturers think that the medical device file is the. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at. Technical File Medical Device Fda.
From www.greenlight.guru
Technical File vs. 510(k) vs. Design History File What Medical Device Technical File Medical Device Fda What are the medical device technical file requirements in iso 13485 and the eu mdr? How should your file be structured and what software will you need? Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical. Technical File Medical Device Fda.
From www.qualitymeddev.com
Medical Device File according to ISO 134852016 QualityMedDev Technical File Medical Device Fda Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Premarket notifications (510(k)), establishment registration, device listing, quality. How should your file be structured and what software will you need? Your technical file must comprehensively demonstrate that the medical device adheres to the general safety. Technical File Medical Device Fda.
From easymedicaldevice.com
How to build a Medical Device Technical Documentation (MDR 2017/745) Technical File Medical Device Fda What are the medical device technical file requirements in iso 13485 and the eu mdr? Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. How should your file be structured and what software will you need? Premarket notifications (510(k)), establishment registration, device listing, quality. Fda has developed this guidance to provide the agency’s. Technical File Medical Device Fda.
From operonstrategist.com
FDA Review Process for 510k Medical Device Submissions Operon Strategist Technical File Medical Device Fda Many manufacturers think that the medical device file is the. How should your file be structured and what software will you need? Premarket notifications (510(k)), establishment registration, device listing, quality. Overview of regulations for medical devices: Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound. Technical File Medical Device Fda.
From www.slideshare.net
US FDA medical device approval chart Emergo Group Technical File Medical Device Fda Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. How should your file be structured and what software will you need? Many manufacturers think that the. Technical File Medical Device Fda.
From www.aplyon.com
CE Marking Procedure Technical File Medical Device Fda Overview of regulations for medical devices: Many manufacturers think that the medical device file is the. Premarket notifications (510(k)), establishment registration, device listing, quality. What are the medical device technical file requirements in iso 13485 and the eu mdr? Iso 13485 requires a medical device file for each medical device type or medical device family. How should your file be. Technical File Medical Device Fda.
From www.greenlight.guru
How to Structure your Medical Device Technical File Technical File Medical Device Fda Overview of regulations for medical devices: Premarket notifications (510(k)), establishment registration, device listing, quality. Iso 13485 requires a medical device file for each medical device type or medical device family. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. How should your file be structured and what software will you need?. Technical File Medical Device Fda.
From matsuda1kawa.blogspot.com
Medical Device Technical File Audit Checklist Technical File Medical Device Fda Premarket notifications (510(k)), establishment registration, device listing, quality. Iso 13485 requires a medical device file for each medical device type or medical device family. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Many manufacturers think that the medical device file is the. Your technical file must comprehensively demonstrate that the. Technical File Medical Device Fda.
From www.kolabtree.com
Medical Device Technical File Checklist The Ultimate Guide Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. Overview of regulations for medical devices: The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Many manufacturers think that the medical device file is the. Fda has developed this guidance to provide the agency’s initial. Technical File Medical Device Fda.
From medicaldevicelicense.com
Complete Technical File Template of Medical Devices for Saudi FDA Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. What are the medical device technical file requirements in iso 13485 and the eu mdr? Fda has developed this guidance to provide the agency’s initial thinking. Technical File Medical Device Fda.
From www.powershow.com
PPT Technical Files for Medical Devices PowerPoint presentation Technical File Medical Device Fda Iso 13485 requires a medical device file for each medical device type or medical device family. Premarket notifications (510(k)), establishment registration, device listing, quality. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at. Technical File Medical Device Fda.
From www.qualio.com
Using Qualio for building a medical device technical file Technical File Medical Device Fda Many manufacturers think that the medical device file is the. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Premarket notifications (510(k)), establishment registration, device listing, quality. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound. Technical File Medical Device Fda.
From easymedicaldevice.com
How to build a Medical Device Technical Documentation (MDR 2017/745) Technical File Medical Device Fda The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. How should your file be structured and what software will you need? Many manufacturers think that the medical device file is the. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. Technical File Medical Device Fda.
From www.simplerqms.com
What is a Medical Device Technical File and How to Structure It? Technical File Medical Device Fda Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Iso 13485 requires a medical device file for each medical. Technical File Medical Device Fda.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Technical File Medical Device Fda Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Iso 13485 requires a medical device file for each medical device type or medical device family. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. What are the. Technical File Medical Device Fda.
From www.youtube.com
European Medical Device Registration Chapter 4 Technical File YouTube Technical File Medical Device Fda The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. Overview of regulations for medical devices: Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Many manufacturers think that the medical device file is the. How should your file be structured and what software will. Technical File Medical Device Fda.
From mavenprofserv.com
Medical Device Technical File Essential Certification Guide Technical File Medical Device Fda Overview of regulations for medical devices: Iso 13485 requires a medical device file for each medical device type or medical device family. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. The file contains detailed information about your medical device, its design, intended use. Technical File Medical Device Fda.
From www.i3cglobal.com
Medical Device Technical File I MDR Technical Documentation Technical File Medical Device Fda How should your file be structured and what software will you need? What are the medical device technical file requirements in iso 13485 and the eu mdr? Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. The file contains detailed information about your medical. Technical File Medical Device Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Technical File Medical Device Fda What are the medical device technical file requirements in iso 13485 and the eu mdr? Overview of regulations for medical devices: Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Your technical file must comprehensively demonstrate that the medical device adheres to the general. Technical File Medical Device Fda.
From www.aplyon.com
Medical Device Report (MDR) Procedure Technical File Medical Device Fda Overview of regulations for medical devices: Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. Many manufacturers think that the medical device file is the. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. The file contains. Technical File Medical Device Fda.
From medicaldeviceacademy.com
Auditing Technical Files Medical Device Academy Technical File Medical Device Fda Premarket notifications (510(k)), establishment registration, device listing, quality. Many manufacturers think that the medical device file is the. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. How should your file be structured and what software will you need? Iso 13485 requires a medical device file for each medical device type or medical. Technical File Medical Device Fda.
From mdlaw.eu
MDR Technical Documentation Guidance · MDlaw Information platform on Technical File Medical Device Fda Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Overview of regulations for medical devices: Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. What are the medical device technical file requirements in iso 13485 and the. Technical File Medical Device Fda.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Technical File Medical Device Fda The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. How should your file be structured and what software will you need? Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Premarket notifications (510(k)), establishment registration, device listing, quality. Many manufacturers think that the medical. Technical File Medical Device Fda.
From www.rimsys.io
FDA 510(k) a beginner's guide Technical File Medical Device Fda Overview of regulations for medical devices: Premarket notifications (510(k)), establishment registration, device listing, quality. Many manufacturers think that the medical device file is the. Iso 13485 requires a medical device file for each medical device type or medical device family. Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing,. Technical File Medical Device Fda.
From www.scribd.com
Preparation of a Technical File Medical Device Documentation Technical File Medical Device Fda Premarket notifications (510(k)), establishment registration, device listing, quality. Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Fda has developed this guidance to provide the agency’s initial thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing. Master files are created to help preserve the trade secrets of. Technical File Medical Device Fda.
From www.youtube.com
TriStar inar Replay Managing FDA Medical Device Compliance in PTC Technical File Medical Device Fda Your technical file must comprehensively demonstrate that the medical device adheres to the general safety and. Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific. What are the medical device technical file requirements in iso 13485 and the eu mdr? Premarket notifications (510(k)), establishment. Technical File Medical Device Fda.