Does Internal Energy Change During Phase Change . Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Understand the concepts of system, surroundings, state function,. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. During a change of phase the temperature does not change, but the internal energy does. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. The internal energy is the sum of the kinetic energy of the molecules and the chemical.
from general.chemistrysteps.com
Learn how energy changes during phase transitions such as melting, boiling, and sublimation. The internal energy is the sum of the kinetic energy of the molecules and the chemical. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Understand the concepts of system, surroundings, state function,. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. During a change of phase the temperature does not change, but the internal energy does. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how.
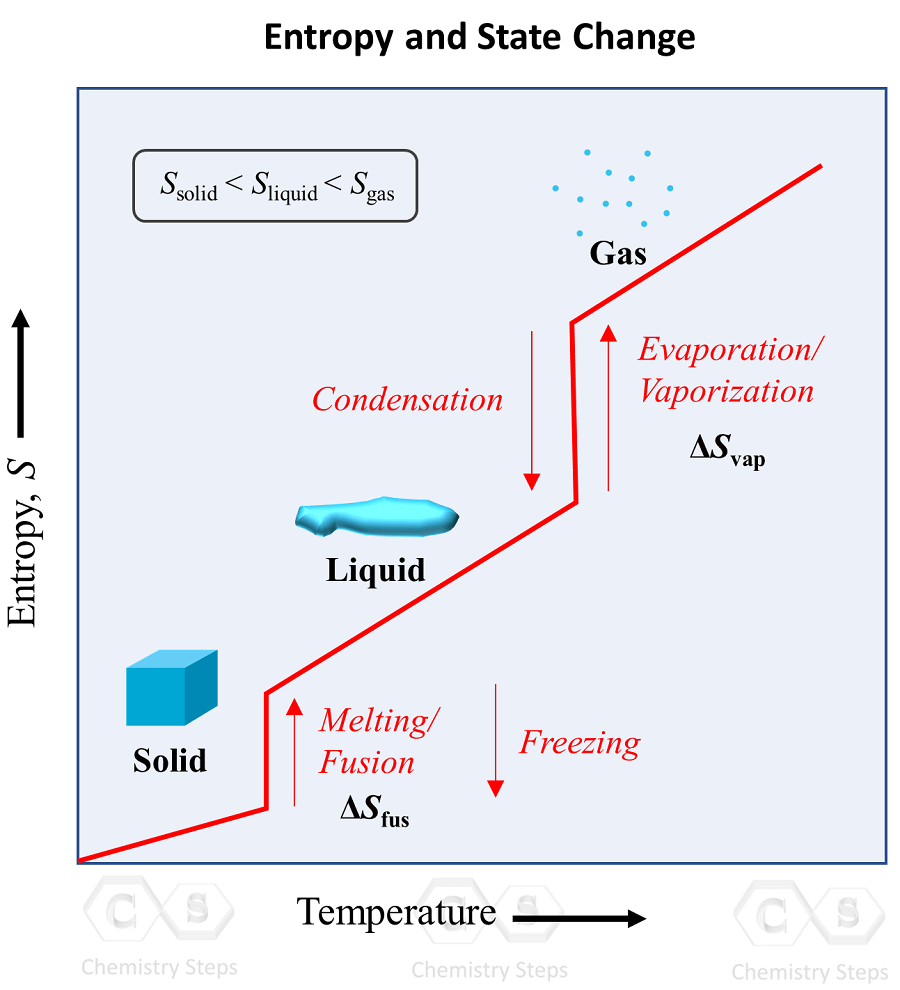
Entropy and State Change Chemistry Steps
Does Internal Energy Change During Phase Change Learn how energy changes during phase transitions such as melting, boiling, and sublimation. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. The internal energy is the sum of the kinetic energy of the molecules and the chemical. During a change of phase the temperature does not change, but the internal energy does. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Understand the concepts of system, surroundings, state function,. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change.
From wiringdatabaseinfo.blogspot.com
Phase Change Diagram With Equations Wiring Site Resource Does Internal Energy Change During Phase Change During a change of phase the temperature does not change, but the internal energy does. The internal energy is the sum of the kinetic energy of the molecules and the chemical. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Plots of pressure. Does Internal Energy Change During Phase Change.
From opentextbc.ca
Phase Changes Basic HVAC Does Internal Energy Change During Phase Change Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. During a change of phase the temperature does not change, but the internal energy does. Understand the concepts of system, surroundings, state function,. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Once water reaches the. Does Internal Energy Change During Phase Change.
From circuitlistgoldschmidt.z19.web.core.windows.net
Phase Change Diagram Explained Does Internal Energy Change During Phase Change During a change of phase the temperature does not change, but the internal energy does. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Understand the concepts of system, surroundings,. Does Internal Energy Change During Phase Change.
From www.youtube.com
Example Using specific heat to calculate ideal gas internal energy Does Internal Energy Change During Phase Change Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. During a change of phase the temperature does not change, but the. Does Internal Energy Change During Phase Change.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Does Internal Energy Change During Phase Change Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. The internal energy is the sum of the kinetic energy of the molecules and the chemical. Once water reaches. Does Internal Energy Change During Phase Change.
From worksheetzonehyland.z19.web.core.windows.net
Heat Due To Phase Change Equations Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Understand the concepts of system, surroundings, state function,. Plots of pressure versus temperatures, an. Does Internal Energy Change During Phase Change.
From www.youtube.com
Including a Phase Change in an Energy Balance YouTube Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Understand the concepts of system, surroundings, state function,. During a change of phase the temperature does not change, but the internal energy. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1549724 Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. During a change of phase the temperature does not change, but the internal energy does. Understand the concepts of system, surroundings, state function,. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. Once water reaches the boiling point, extra. Does Internal Energy Change During Phase Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Does Internal Energy Change During Phase Change During a change of phase the temperature does not change, but the internal energy does. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Learn how energy changes during phase transitions such. Does Internal Energy Change During Phase Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Does Internal Energy Change During Phase Change Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. Understand the concepts of system, surroundings, state function,. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal. Does Internal Energy Change During Phase Change.
From sciencepickle.com
Latent Heat Science Pickle Does Internal Energy Change During Phase Change Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. The internal energy is the sum of the kinetic energy of the molecules and the chemical. The internal energy is the sum of. Does Internal Energy Change During Phase Change.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula Does Internal Energy Change During Phase Change Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Understand the concepts of system, surroundings, state function,. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. The internal energy is the sum of the kinetic energy of the molecules. Does Internal Energy Change During Phase Change.
From www.youtube.com
Energy calculation during phase change YouTube Does Internal Energy Change During Phase Change Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Understand the concepts of system, surroundings, state function,. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction.. Does Internal Energy Change During Phase Change.
From guides.co
Phase Change Requires Heat FD2021 Fundamentals of Fire and Does Internal Energy Change During Phase Change During a change of phase the temperature does not change, but the internal energy does. Understand the concepts of system, surroundings, state function,. The internal energy is the sum of the kinetic energy of the molecules and the chemical. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy. Does Internal Energy Change During Phase Change.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. During a change of phase the temperature does not change, but the internal energy does. Plots of pressure versus temperatures, an example of. Does Internal Energy Change During Phase Change.
From www.youtube.com
Change in Internal Energy Using Specific Heat in 3 Minutes! YouTube Does Internal Energy Change During Phase Change Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Find. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3746915 Does Internal Energy Change During Phase Change Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Understand the concepts of system, surroundings, state function,. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. The internal energy. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT to CHEMISTRY !!! PowerPoint Presentation, free download Does Internal Energy Change During Phase Change Learn how energy changes during phase transitions such as melting, boiling, and sublimation. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Explain how internal energy is related to the phase. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Does Internal Energy Change During Phase Change Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. The internal energy is the sum of the kinetic energy of the molecules and the chemical. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT States of Matter & Phase Changes PowerPoint Presentation ID6286577 Does Internal Energy Change During Phase Change The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. During a change of phase the temperature does not change, but the internal energy does. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. If $\delta h$ for. Does Internal Energy Change During Phase Change.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps Does Internal Energy Change During Phase Change The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. During a phase. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free Does Internal Energy Change During Phase Change Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of. Does Internal Energy Change During Phase Change.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Does Internal Energy Change During Phase Change Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. The internal energy. Does Internal Energy Change During Phase Change.
From quizzlistreplevies.z13.web.core.windows.net
Heat Energy During Phase Change Does Internal Energy Change During Phase Change If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential. Does Internal Energy Change During Phase Change.
From galvinconanstuart.blogspot.com
Phase Change Diagram With Equations General Wiring Diagram Does Internal Energy Change During Phase Change Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Understand the concepts of system, surroundings, state function,. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Find out the enthalpies of fusion, vaporization, and. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Energy and Phase Changes PowerPoint Presentation, free download Does Internal Energy Change During Phase Change If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. The internal energy is the sum of the kinetic energy of the molecules and the chemical.. Does Internal Energy Change During Phase Change.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Does Internal Energy Change During Phase Change Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. The internal energy is the sum of the kinetic energy of the molecules and the chemical. If $\delta h$ for the. Does Internal Energy Change During Phase Change.
From gazemoms.blogspot.com
The Internal Energy Of A System Is Always Increased By gazemoms Does Internal Energy Change During Phase Change If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Explain how internal energy is related to the phase changes. Does Internal Energy Change During Phase Change.
From www.youtube.com
Calculating the Energy of a Phase Change YouTube Does Internal Energy Change During Phase Change Learn how to calculate changes in internal energy and the flow of energy during a chemical reaction. During a change of phase the temperature does not change, but the internal energy does. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. The internal energy is the sum of the kinetic energy. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation, free Does Internal Energy Change During Phase Change The internal energy is the sum of the kinetic energy of the molecules and the chemical. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. The internal energy is the sum of the kinetic energy of the molecules. Does Internal Energy Change During Phase Change.
From slideplayer.com
Energy Changes & Phase Changes ppt download Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. Learn how energy changes during phase transitions such as melting, boiling, and sublimation. Understand the concepts of system, surroundings, state function,. If $\delta h$ for the transition of ice to water is $\mathrm{1440~cal}$, calculate the change in internal energy. Plots. Does Internal Energy Change During Phase Change.
From www.dynamicscience.com.au
Chemistrylatent heatphase change diagrams Does Internal Energy Change During Phase Change Explain how internal energy is related to the phase changes of a substance, and why temperature remains constant during a phase change. Understand the concepts of system, surroundings, state function,. During a change of phase the temperature does not change, but the internal energy does. Learn how to calculate changes in internal energy and the flow of energy during a. Does Internal Energy Change During Phase Change.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Does Internal Energy Change During Phase Change During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. The internal energy is the sum of the kinetic energy of the molecules and the chemical. During a change of phase the temperature does not change, but the internal energy does. The internal energy is the sum of the kinetic. Does Internal Energy Change During Phase Change.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID4342222 Does Internal Energy Change During Phase Change Plots of pressure versus temperatures, an example of a phase diagram, provide considerable insight into thermal properties of substances. Find out the enthalpies of fusion, vaporization, and sublimation for selected substances and how. During a phase change, internal energy of a system will change because energy must be added or subtracted (depending on. During a change of phase the temperature. Does Internal Energy Change During Phase Change.