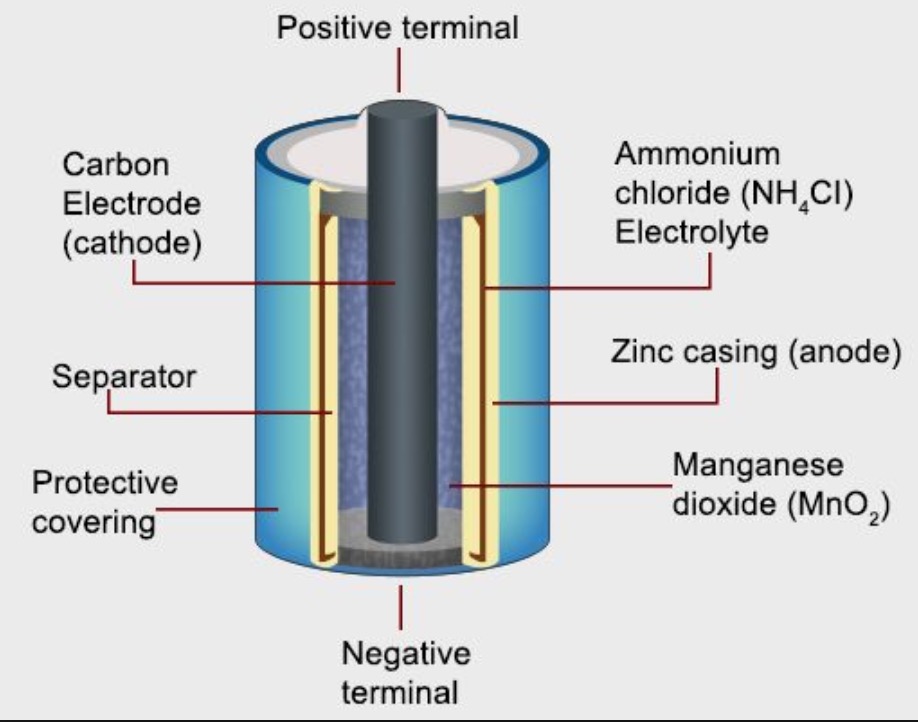
Battery Electrochemical Cell . batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. a battery is an electrochemical cell or series of cells that produces an electric current. A clearer picture of basic electrochemistry emerges from this energy analysis. In principle, any galvanic cell could be used. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is a device that stores chemical energy, and converts it to electricity. Each electrochemical cell consists of two.
from exoojhjhg.blob.core.windows.net
in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. A clearer picture of basic electrochemistry emerges from this energy analysis. Each electrochemical cell consists of two. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. a battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is a device that stores chemical energy, and converts it to electricity. A battery can be made up of one or several (like in volta's original pile) electrochemical cells.
Battery Electrolyte Dry at Daniel Nagle blog
Battery Electrochemical Cell batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. Each electrochemical cell consists of two. A clearer picture of basic electrochemistry emerges from this energy analysis. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. a battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery is a device that stores chemical energy, and converts it to electricity.
From saylordotorg.github.io
Electrochemistry Battery Electrochemical Cell a battery is a device that stores chemical energy, and converts it to electricity. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. Each electrochemical cell consists of two. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. in. Battery Electrochemical Cell.
From ul.org
What Are LithiumIon Batteries? UL Research Institutes Battery Electrochemical Cell a battery is a device that stores chemical energy, and converts it to electricity. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. A clearer picture of basic electrochemistry emerges from this energy analysis. Each electrochemical cell consists of two. This is known as electrochemistry and the system. Battery Electrochemical Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Battery Electrochemical Cell In principle, any galvanic cell could be used. a battery is a device that stores chemical energy, and converts it to electricity. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. an extremely. Battery Electrochemical Cell.
From electrochemistryresources.com
Batteries / Electrochemical Cells Battery Electrochemical Cell Each electrochemical cell consists of two. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. a battery is a device that stores chemical energy, and converts it to electricity. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery. Battery Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Battery Electrochemical Cell an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. In principle, any galvanic cell could be used. Each electrochemical cell consists of two. a battery is a device that stores chemical energy, and converts it to electricity. batteries are composed of at least one electrochemical cell which is. Battery Electrochemical Cell.
From www.electricity-magnetism.org
Composition of Battery Parts of Battery Electricity Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to. Battery Electrochemical Cell.
From www.britannica.com
Battery Primary Cells, Rechargeable, Chemistry Britannica Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. a battery is an electrochemical cell or series of cells that produces an electric current. Each electrochemical cell consists of two. A battery. Battery Electrochemical Cell.
From www.sliderbase.com
Electrochemical Terminology Battery Electrochemical Cell In principle, any galvanic cell could be used. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. A clearer picture of basic electrochemistry emerges from this energy analysis. in a battery. Battery Electrochemical Cell.
From saylordotorg.github.io
Commercial Galvanic Cells Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. A clearer picture of basic electrochemistry emerges from this energy analysis. a battery is a device that stores chemical energy, and converts it. Battery Electrochemical Cell.
From www.researchgate.net
(a) Photograph of the customized electrochemical cell used for zinc−air Battery Electrochemical Cell Each electrochemical cell consists of two. a battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. A clearer picture of basic electrochemistry emerges from this energy. Battery Electrochemical Cell.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Battery Electrochemical Cell A battery can be made up of one or several (like in volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. a battery is an electrochemical. Battery Electrochemical Cell.
From www.researchgate.net
8 Typical electrochemical battery cell. Download Scientific Diagram Battery Electrochemical Cell A clearer picture of basic electrochemistry emerges from this energy analysis. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery is a device that stores chemical energy, and converts it to electricity. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy. Battery Electrochemical Cell.
From www.researchgate.net
Schematic representation of a Liion battery cell. Download Battery Electrochemical Cell A clearer picture of basic electrochemistry emerges from this energy analysis. In principle, any galvanic cell could be used. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. a. Battery Electrochemical Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is a device that stores chemical energy, and converts it to electricity. Each electrochemical cell consists of two. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. In principle, any galvanic cell. Battery Electrochemical Cell.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Battery Electrochemical Cell A clearer picture of basic electrochemistry emerges from this energy analysis. In principle, any galvanic cell could be used. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. A battery. Battery Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Battery Electrochemical Cell Each electrochemical cell consists of two. a battery is a device that stores chemical energy, and converts it to electricity. a battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used. in a battery (also known as a galvanic cell), current is produced when electrons. Battery Electrochemical Cell.
From www.thoughtco.com
Electrochemical Cell Definition Battery Electrochemical Cell Each electrochemical cell consists of two. a battery is an electrochemical cell or series of cells that produces an electric current. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. In principle, any galvanic. Battery Electrochemical Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Battery Electrochemical Cell Each electrochemical cell consists of two. In principle, any galvanic cell could be used. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. in a battery (also known as a galvanic cell),. Battery Electrochemical Cell.
From www.vrogue.co
Consider The Following Electrochemical Cell vrogue.co Battery Electrochemical Cell Each electrochemical cell consists of two. a battery is a device that stores chemical energy, and converts it to electricity. In principle, any galvanic cell could be used. a battery is an electrochemical cell or series of cells that produces an electric current. A battery can be made up of one or several (like in volta's original pile). Battery Electrochemical Cell.
From www.researchgate.net
P2D electrochemical model representation of Liion battery. Download Battery Electrochemical Cell In principle, any galvanic cell could be used. a battery is a device that stores chemical energy, and converts it to electricity. a battery is an electrochemical cell or series of cells that produces an electric current. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. Each electrochemical cell consists. Battery Electrochemical Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Battery Electrochemical Cell In principle, any galvanic cell could be used. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. a battery is an electrochemical cell or series of cells that produces an electric current. A battery can be made up of one or several (like in volta's original pile) electrochemical. Battery Electrochemical Cell.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Battery Electrochemical Cell a battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used. A clearer picture of basic electrochemistry emerges from this energy analysis. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is a device that stores. Battery Electrochemical Cell.
From www.researchgate.net
Schematic of lithium ion battery electrochemical model.... Download Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. batteries are composed of at least one electrochemical cell which is used for the storage. Battery Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Battery Electrochemical Cell in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. a battery is a device that stores chemical energy, and converts it to electricity. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. Battery Electrochemical Cell.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is an electrochemical cell or series of cells that produces an electric current. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. A clearer picture of basic electrochemistry emerges from this energy. Battery Electrochemical Cell.
From www.ourfutureenergy.co.uk
Light from salt OurFuture.Energy Battery Electrochemical Cell In principle, any galvanic cell could be used. A clearer picture of basic electrochemistry emerges from this energy analysis. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. a battery is a device that stores chemical energy, and converts it to electricity. batteries are composed of at least. Battery Electrochemical Cell.
From exoojhjhg.blob.core.windows.net
Battery Electrolyte Dry at Daniel Nagle blog Battery Electrochemical Cell a battery is a device that stores chemical energy, and converts it to electricity. in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. . Battery Electrochemical Cell.
From www.youtube.com
How does a battery work? Electrochemical cells, redox, MgCu cell in Battery Electrochemical Cell in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. an extremely important class of oxidation and reduction reactions are used to provide useful electrical. Battery Electrochemical Cell.
From www.electroniclinic.com
What is battery? Types of battery, Primary and Secondary cells Battery Electrochemical Cell Each electrochemical cell consists of two. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. In principle, any galvanic cell could be used. A battery can be made up of one or several. Battery Electrochemical Cell.
From studylib.net
Electrochemical Cells (Batteries) Battery Electrochemical Cell a battery is an electrochemical cell or series of cells that produces an electric current. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. A clearer picture of basic electrochemistry emerges from this energy analysis. Each electrochemical cell consists of two. an extremely important class of oxidation and reduction reactions. Battery Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Battery Electrochemical Cell A clearer picture of basic electrochemistry emerges from this energy analysis. a battery is a device that stores chemical energy, and converts it to electricity. In principle, any galvanic cell could be used. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. in a battery (also known. Battery Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Battery Electrochemical Cell a battery is a device that stores chemical energy, and converts it to electricity. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. In principle, any galvanic cell could be used. A clearer picture of basic electrochemistry emerges from this energy analysis. a battery is an electrochemical cell. Battery Electrochemical Cell.
From mavink.com
Electrochemical Cell Diagram Battery Electrochemical Cell This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. an extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. A clearer picture of basic electrochemistry emerges. Battery Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Battery Electrochemical Cell in a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance. batteries are composed of at least one electrochemical cell which is used for the storage and generation of electricity. This is known as electrochemistry and the system that underpins a battery is. Battery Electrochemical Cell.
From www.slideserve.com
PPT Electrochemical Cell PowerPoint Presentation, free download ID Battery Electrochemical Cell Each electrochemical cell consists of two. a battery is an electrochemical cell or series of cells that produces an electric current. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. A clearer picture of basic electrochemistry emerges from this energy analysis. A battery can be made up of one or several. Battery Electrochemical Cell.