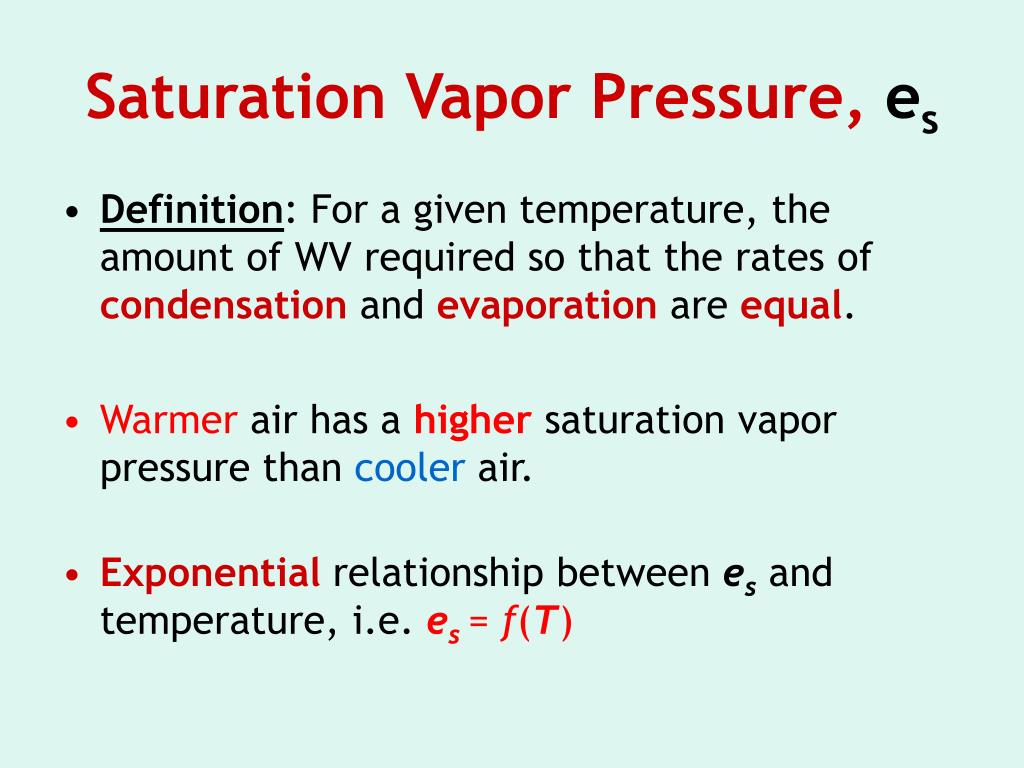
Why Does Saturation Vapor Pressure Increase With Temperature . It also looks at how saturated vapour pressure. How does saturated vapour pressure relate to vapour pressure? There are four terms you need to understand. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It needs heat to convert the liquid into the vapor. Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure.
from www.slideserve.com
According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. How does saturated vapour pressure relate to vapour pressure? It needs heat to convert the liquid into the vapor. It also looks at how saturated vapour pressure. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. There are four terms you need to understand. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for.
PPT AOS 101304 PowerPoint Presentation, free download ID4666349
Why Does Saturation Vapor Pressure Increase With Temperature It needs heat to convert the liquid into the vapor. There are four terms you need to understand. How does saturated vapour pressure relate to vapour pressure? This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It needs heat to convert the liquid into the vapor. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for.
From www.vrogue.co
P V Diagram P V Diagram Pressure Ver Ygraph vrogue.co Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the liquid into the vapor. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Saturation Vapor Pressure Increase With Temperature For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. There are four terms you need to understand. Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the liquid into the vapor. How does saturated vapour pressure relate to vapour pressure? This page looks at how. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
A. Relationship between vapor pressure, relative humidity and Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It needs heat to convert the. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure of methanol and ethanol as a function of temperature Why Does Saturation Vapor Pressure Increase With Temperature It also looks at how saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
The saturation curve of pressure against temperature for chlorine Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the liquid into the vapor. There are four terms you need to understand. It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Why Does Saturation Vapor Pressure Increase With Temperature There are four terms you need to understand. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. How does saturated vapour pressure relate to vapour pressure? For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. According. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Presentation Slides for Chapter 2 of Fundamentals of Atmospheric Why Does Saturation Vapor Pressure Increase With Temperature For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. There are four terms you need to understand. How does saturated vapour pressure relate to vapour pressure? Since. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.engineeringtoolbox.com
Water Saturation Pressure vs. Temperature Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. How does saturated vapour pressure relate to vapour pressure? Since the vapor pressure increases with temperature, it follows that for. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED Over a water surface, the saturation vapor pressure (es) is Why Does Saturation Vapor Pressure Increase With Temperature It needs heat to convert the liquid into the vapor. There are four terms you need to understand. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. How does saturated vapour pressure relate to vapour pressure? According to le chatelier, increasing the temperature of. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the liquid into the vapor. There are four terms you need to understand. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. It also looks at how saturated vapour pressure. According to le chatelier, increasing the. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
Saturation pressure as a function of the saturation temperature for Why Does Saturation Vapor Pressure Increase With Temperature According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. How does saturated vapour pressure relate to vapour pressure? It needs heat to convert the liquid into the vapor. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. Since the vapor pressure increases with. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure and density of water vapor as function of temperature Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. Since the vapor pressure increases with temperature, it follows that for. There are four terms you need to. Why Does Saturation Vapor Pressure Increase With Temperature.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. It also looks at how saturated vapour pressure. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated. Why Does Saturation Vapor Pressure Increase With Temperature.
From pressbooks.bccampus.ca
2.3 Phase diagrams Introduction to Engineering Thermodynamics Why Does Saturation Vapor Pressure Increase With Temperature According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. There are four terms you need to understand. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. How does saturated vapour pressure relate to vapour pressure?. Why Does Saturation Vapor Pressure Increase With Temperature.
From chart-studio.plotly.com
Saturation Vapor Pressure of Propane as a Function of Temperature Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. There are four terms you need to understand. How does saturated vapour pressure relate to vapour pressure? This page looks at how the equilibrium between a liquid (or a solid) and its. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved The vapor pressure behavior of two liquids, methanol Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It also looks at how saturated vapour pressure. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the. Why Does Saturation Vapor Pressure Increase With Temperature.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Why Does Saturation Vapor Pressure Increase With Temperature It needs heat to convert the liquid into the vapor. It also looks at how saturated vapour pressure. There are four terms you need to understand. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. This page looks at how the equilibrium between a liquid (or a solid) and its vapour. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved You are given the graph of the saturation vapor Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. For water, the vapor pressure reaches the standard sea. Why Does Saturation Vapor Pressure Increase With Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the liquid into the vapor. How does saturated vapour pressure relate to vapour pressure? This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. There are four terms you. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It also looks at how saturated vapour pressure. There are four terms you need to understand. It needs heat to convert the liquid into the vapor. For water, the vapor pressure reaches the standard sea. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Water Vapor, Clouds, and Precipitation PowerPoint Presentation Why Does Saturation Vapor Pressure Increase With Temperature According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. How does saturated vapour pressure relate to vapour pressure? There are four terms you need to understand. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. Since the vapor pressure increases with temperature, it. Why Does Saturation Vapor Pressure Increase With Temperature.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Why Does Saturation Vapor Pressure Increase With Temperature For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. There are four terms you need to understand. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Precipitation I PowerPoint Presentation, free download ID6891455 Why Does Saturation Vapor Pressure Increase With Temperature This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It also looks at how saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at. Why Does Saturation Vapor Pressure Increase With Temperature.
From guidelibunveracity.z21.web.core.windows.net
Temperature Entropy Diagram For Steam Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. This page looks at how the. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Why Does Saturation Vapor Pressure Increase With Temperature For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Humidity and Condensation PowerPoint Presentation, free download Why Does Saturation Vapor Pressure Increase With Temperature How does saturated vapour pressure relate to vapour pressure? There are four terms you need to understand. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. This page looks at how the equilibrium between. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
Correlation between temperature and saturation vapor pressure [4 Why Does Saturation Vapor Pressure Increase With Temperature There are four terms you need to understand. Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. It also looks at how saturated vapour pressure. How does saturated vapour pressure relate to vapour pressure? According to le chatelier, increasing the temperature. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.chemistrylearner.com
Saturated Vapor Pressure Definition and Temperature Effect Why Does Saturation Vapor Pressure Increase With Temperature According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. How does saturated vapour pressure relate to vapour pressure? There are four terms you need to understand. It needs heat to convert the liquid into. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.researchgate.net
The saturated vapor pressure versus saturation temperature of HFC125 Why Does Saturation Vapor Pressure Increase With Temperature It needs heat to convert the liquid into the vapor. How does saturated vapour pressure relate to vapour pressure? This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 4 PROPERTIES OF PURE SUBSTANCES PowerPoint Presentation Why Does Saturation Vapor Pressure Increase With Temperature For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Since the vapor pressure increases with temperature, it follows that for. According to le chatelier, increasing the temperature. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Why Does Saturation Vapor Pressure Increase With Temperature It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It needs heat to convert the liquid into the vapor. How does saturated vapour pressure relate to vapour pressure? According to le chatelier, increasing the temperature of. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.youtube.com
Saturation Pressure from EOS Spreadsheet YouTube Why Does Saturation Vapor Pressure Increase With Temperature It needs heat to convert the liquid into the vapor. How does saturated vapour pressure relate to vapour pressure? There are four terms you need to understand. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. It also looks at how saturated vapour pressure. Since the vapor pressure increases with temperature, it. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved The saturation vapor pressure is a function of Why Does Saturation Vapor Pressure Increase With Temperature How does saturated vapour pressure relate to vapour pressure? According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It needs heat to convert the liquid into the vapor. It also looks at how saturated vapour pressure. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Moisture Relationships PowerPoint Presentation, free download Why Does Saturation Vapor Pressure Increase With Temperature Since the vapor pressure increases with temperature, it follows that for. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. It needs heat to convert the liquid into the vapor. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a. Why Does Saturation Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT AOS 101304 PowerPoint Presentation, free download ID4666349 Why Does Saturation Vapor Pressure Increase With Temperature According to le chatelier, increasing the temperature of a system in a dynamic equilibrium favors the endothermic change. It also looks at how saturated vapour pressure. For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mmhg at 100°c. Since the vapor pressure increases with temperature, it follows that for. It needs heat to convert the. Why Does Saturation Vapor Pressure Increase With Temperature.