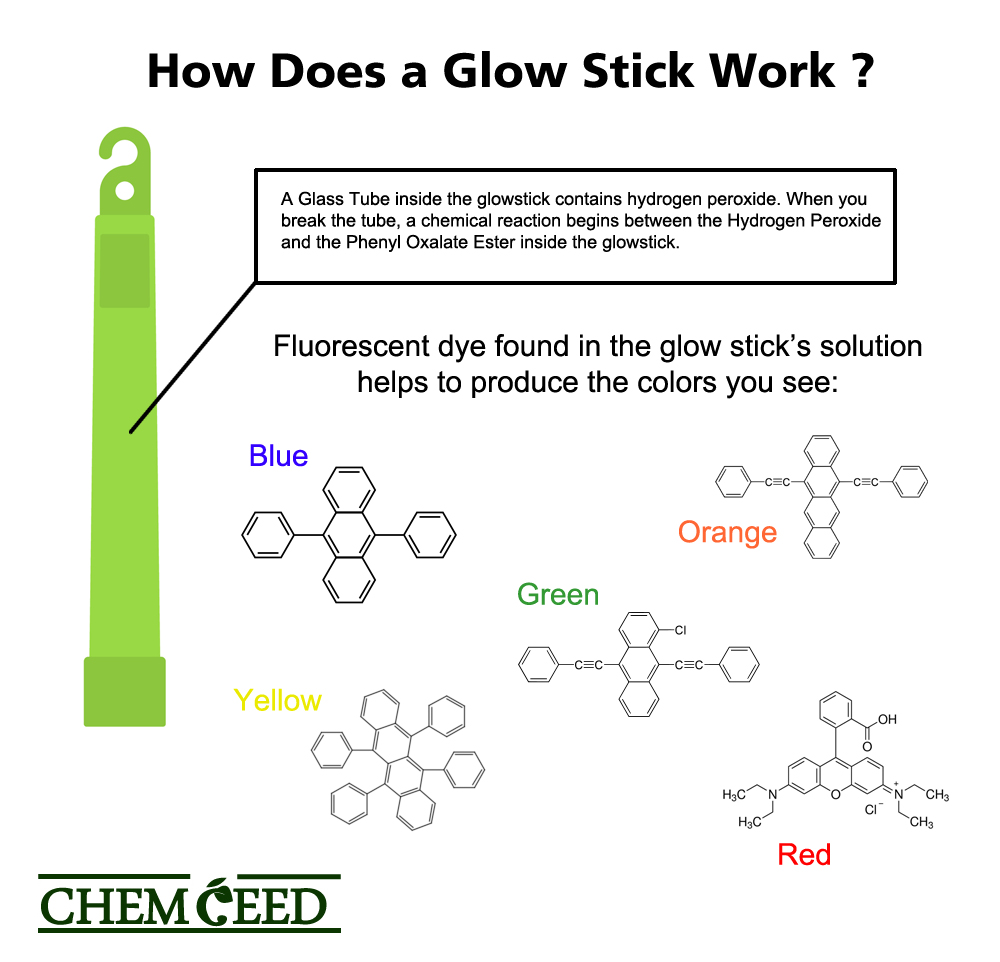
How Does Luminol Work . The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. Oxidation of luminol produces excited 3. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. To exhibit its luminescence, the luminol must first be activated with an oxidant.
from chemceed.com
Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light.
The Science of Chem Light ChemCeed
How Does Luminol Work Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. Oxidation of luminol produces excited 3. To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light.
From fyoiopvvh.blob.core.windows.net
How Does Luminol Detect Blood at Isaac Hensley blog How Does Luminol Work The excited state of the luminol than changes back to the stable state with the emission of light. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do. How Does Luminol Work.
From studylib.net
luminol1 How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. To exhibit its luminescence, the luminol must first be activated with an oxidant. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation, free download ID How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. To exhibit its luminescence, the luminol must first be activated with an oxidant. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol’s blue colour can be modified to more of a yellowy green by. How Does Luminol Work.
From slideplayer.com
DNA FORENSICS How does forensic identification work? ppt video online How Does Luminol Work Oxidation of luminol produces excited 3. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation ID4209844 How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The excited state of the luminol than changes back to the stable state with the emission of light. The luminol is oxidized by the hyrogen peroxide in a basic solution to an. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation, free download ID How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The excited state of the luminol than changes back to the stable state with the emission of light. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol’s blue colour can be modified to more of a yellowy. How Does Luminol Work.
From gbu-presnenskij.ru
Luminol And Hydrogen Peroxide Reaction Buying Store gbupresnenskij.ru How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be. How Does Luminol Work.
From www.slideserve.com
PPT Luminol and Bluestar PowerPoint Presentation, free download ID How Does Luminol Work Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Oxidation of luminol produces. How Does Luminol Work.
From www.youtube.com
Luminol Test How the Luminol Reaction works with Blood YouTube How Does Luminol Work The excited state of the luminol than changes back to the stable state with the emission of light. To exhibit its luminescence, the luminol must first be activated with an oxidant. Oxidation of luminol produces excited 3. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide. How Does Luminol Work.
From www.compoundchem.com
Crime Scene Chemistry Luminol, Blood & Horseradish Compound Interest How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol’s blue colour can be. How Does Luminol Work.
From deshengbio.en.made-in-china.com
How Does Luminol Work Emit Blue Light When Mixed with Oxidants China How Does Luminol Work The excited state of the luminol than changes back to the stable state with the emission of light. To exhibit its luminescence, the luminol must first be activated with an oxidant. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol is a reagent that detects substances with peroxidase activity, one of which. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation, free download ID How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol’s blue colour can be modified to more of a yellowy green by. How Does Luminol Work.
From www.bio-rad.com
ECL western blotting substrates for horseradish peroxidase (HRP How Does Luminol Work Oxidation of luminol produces excited 3. The excited state of the luminol than changes back to the stable state with the emission of light. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks. How Does Luminol Work.
From www.slideserve.com
PPT Blood stain analysis PowerPoint Presentation, free download ID How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates. How Does Luminol Work.
From www.youtube.com
luminol demo HD YouTube How Does Luminol Work Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. The excited state of the luminol than changes back to the stable state with the emission of light. The. How Does Luminol Work.
From www.slideshare.net
Chemiluminescence in blood stain detection ppt How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. To exhibit its luminescence, the luminol must first be activated with an oxidant. The excited state of the luminol than changes back to. How Does Luminol Work.
From www.lihpao.com
How Does Luminol Work? A Comprehensive Guide to Its Uses in Crime Scene How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that. How Does Luminol Work.
From www.youtube.com
How to determine Blood Traces The Fluorescent Chemistry of Luminol How Does Luminol Work Oxidation of luminol produces excited 3. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. To exhibit its luminescence, the. How Does Luminol Work.
From www.vectorstock.com
Luminol chemical chemiluminescence Royalty Free Vector Image How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. Luminol is a. How Does Luminol Work.
From slideplayer.com
Chemiluminescence Sam & Tina . ppt download How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Oxidation of luminol produces excited 3. To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that. How Does Luminol Work.
From physicsopenlab.org
Luminol PhysicsOpenLab How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. Oxidation of luminol produces excited 3. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The excited state of the luminol than changes back to. How Does Luminol Work.
From www.youtube.com
The Luminol Reaction YouTube How Does Luminol Work Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. To exhibit its luminescence, the luminol must first be activated with an oxidant. Oxidation of luminol produces excited 3.. How Does Luminol Work.
From www.tradewheel.com
Buy How Luminol Works from HUBEI NEW DESHENG MATERIALS TECHNOLOGY CO How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The excited state of the luminol than changes back to the stable state with the emission. How Does Luminol Work.
From www.youtube.com
How Luminol Works Chemiluminescence YouTube How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. To exhibit its luminescence, the luminol must first be activated with. How Does Luminol Work.
From www.slideserve.com
PPT Luminol Structure, Synthesis, chemical reaction, and its How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to. How Does Luminol Work.
From chemceed.com
The Science of Chem Light ChemCeed How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. To exhibit its. How Does Luminol Work.
From www.lihpao.com
How Does Luminol Work? A Comprehensive Guide to Its Uses in Crime Scene How Does Luminol Work The excited state of the luminol than changes back to the stable state with the emission of light. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. The. How Does Luminol Work.
From www.slideserve.com
PPT Luminol and Bluestar PowerPoint Presentation, free download ID How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation. How Does Luminol Work.
From www.slideserve.com
PPT What are the characteristics/traits of a good forensic DNA How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. Luminol is a reagent that detects. How Does Luminol Work.
From www.youtube.com
The Pulsing Glow Stick Oscillating Luminol Clock Reaction YouTube How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. The excited state of. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation ID4209844 How Does Luminol Work To exhibit its luminescence, the luminol must first be activated with an oxidant. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. Oxidation of luminol produces excited 3.. How Does Luminol Work.
From www.slideserve.com
PPT LUMINOL & BLUESTAR PowerPoint Presentation, free download ID How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Oxidation of luminol produces excited 3. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation. How Does Luminol Work.
From www.slideshare.net
Group 5 luminol & dna How Does Luminol Work Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that do not themselves give off visible light but rather pass energy to dyes or ‘sensitisers’. To exhibit its luminescence,. How Does Luminol Work.
From www.pinterest.com
Pin on Chemistry How Does Luminol Work The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. The excited state of the luminol than changes back to the stable state with the emission of light. Oxidation of luminol produces excited 3. Luminol is a reagent that detects substances with peroxidase activity, one of which is blood. To exhibit its luminescence, the. How Does Luminol Work.
From www.lihpao.com
How Does Luminol Work? A Comprehensive Guide to Its Uses in Crime Scene How Does Luminol Work The excited state of the luminol than changes back to the stable state with the emission of light. The luminol is oxidized by the hyrogen peroxide in a basic solution to an excited state. Luminol’s blue colour can be modified to more of a yellowy green by masking with fluorescein, but commercial glow sticks involve the generation of intermediates that. How Does Luminol Work.