Factor Calculation For Titration . a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. calculating ph for titration solutions: our titration calculator will help you never have to ask how do i calculate titrations?.
from general.chemistrysteps.com
calculating ph for titration solutions: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. our titration calculator will help you never have to ask how do i calculate titrations?. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
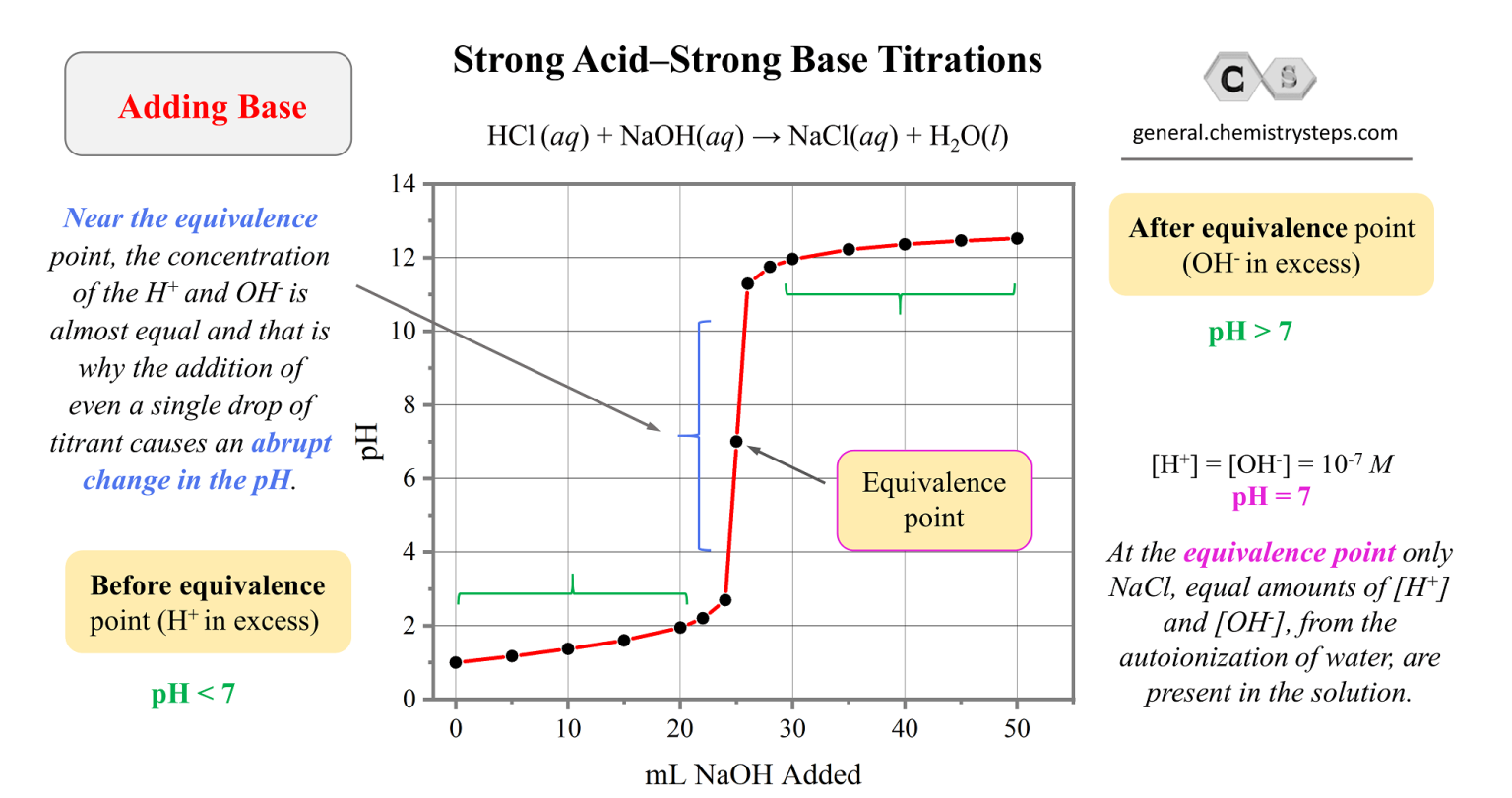
Strong AcidStrong Base Titrations Chemistry Steps
Factor Calculation For Titration in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. our titration calculator will help you never have to ask how do i calculate titrations?. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. calculating ph for titration solutions:
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Factor Calculation For Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is used to find an unknown concentration of. Factor Calculation For Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Factor Calculation For Titration a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. At the equivalence point in a neutralization, the moles of acid are equal to. Factor Calculation For Titration.
From exonhrnmo.blob.core.windows.net
Titration Formula Ratio at Sarah Campbell blog Factor Calculation For Titration in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. our titration calculator will help you never have to ask how do i calculate titrations?. . Factor Calculation For Titration.
From learningschoolmurgkerny1.z13.web.core.windows.net
Chemistry Titration Questions And Answers Factor Calculation For Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Factor Calculation For Titration.
From learningschoolregibanb.z21.web.core.windows.net
Chemistry Titration Questions And Answers Factor Calculation For Titration calculating ph for titration solutions: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. our titration calculator will help you never have to ask. Factor Calculation For Titration.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. . Factor Calculation For Titration.
From www.researchgate.net
The sample, dilution factor, and BOD values and the calculated degrees Factor Calculation For Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculating ph for titration solutions: the aim of this booklet is. Factor Calculation For Titration.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Factor Calculation For Titration the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Factor Calculation For Titration.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Factor Calculation For Titration calculating ph for titration solutions: a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant. Factor Calculation For Titration.
From dxojrsulq.blob.core.windows.net
How To Report A Titration Experiment at Joseph Heitmann blog Factor Calculation For Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. the aim of this booklet is to explore titration from. Factor Calculation For Titration.
From www.youtube.com
DilutionTitration Calculation ChemCram 101 Tutorial YouTube Factor Calculation For Titration in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. calculating ph for titration solutions: the aim of this booklet is. Factor Calculation For Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing. Factor Calculation For Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Factor Calculation For Titration calculating ph for titration solutions: in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the.. Factor Calculation For Titration.
From learningschoolregibanb.z21.web.core.windows.net
How To Do Titrations In Chemistry Factor Calculation For Titration in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. calculating ph for titration solutions: the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. a titration is used. Factor Calculation For Titration.
From mungfali.com
Acid Base Titration Calculation Factor Calculation For Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Factor Calculation For Titration.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. . Factor Calculation For Titration.
From www.youtube.com
A short guide to titration calculations YouTube Factor Calculation For Titration calculating ph for titration solutions: our titration calculator will help you never have to ask how do i calculate titrations?. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is used to find an unknown concentration of a solution by reacting it with a. Factor Calculation For Titration.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Factor Calculation For Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. At the equivalence point in a neutralization,. Factor Calculation For Titration.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Factor Calculation For Titration the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. At the equivalence point in a neutralization, the moles of acid are equal to. Factor Calculation For Titration.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Factor Calculation For Titration calculating ph for titration solutions: the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles of acid are equal to. Factor Calculation For Titration.
From exosjwdrb.blob.core.windows.net
Titration Calculation Table Method at Susan Clyburn blog Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. our titration calculator will help you never have to ask how do i calculate titrations?. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. . Factor Calculation For Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. a titration is used to find an unknown concentration of a solution by reacting it with. Factor Calculation For Titration.
From www.youtube.com
Normality Factor 08 Titration Class 11th & 12th YouTube Factor Calculation For Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. At the equivalence point in a neutralization, the moles of acid. Factor Calculation For Titration.
From www.science-revision.co.uk
Titrations Factor Calculation For Titration calculating ph for titration solutions: a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. At the equivalence point in a neutralization,. Factor Calculation For Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID234018 Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Factor Calculation For Titration.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Factor Calculation For Titration calculating ph for titration solutions: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a.. Factor Calculation For Titration.
From dxoeafgyd.blob.core.windows.net
Titration Formula Gcse at Doris Guyer blog Factor Calculation For Titration calculating ph for titration solutions: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles. Factor Calculation For Titration.
From www.chegg.com
Karl Fischer Titration Assignment Calculation Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100. Factor Calculation For Titration.
From cevfuhpn.blob.core.windows.net
Titration Percentage Formula at Grace Sasso blog Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. the aim of this booklet is to explore titration from a historical, theoretical and practical point of view, dealing first with preset end. a titration is used to find an unknown concentration of a solution by reacting it with a solution of. Factor Calculation For Titration.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. our titration calculator will help you never have to ask how do i calculate titrations?. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is carried. Factor Calculation For Titration.
From www.pinterest.com
Pin on Chemistry Factor Calculation For Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Factor Calculation For Titration.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. calculating ph for titration solutions: a titration is used to find an unknown concentration of a solution. Factor Calculation For Titration.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Factor Calculation For Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. a titration is used to. Factor Calculation For Titration.
From www.youtube.com
Acids and Bases Back Titration Calculation Exam Question|A Level Factor Calculation For Titration our titration calculator will help you never have to ask how do i calculate titrations?. calculating ph for titration solutions: a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant). Factor Calculation For Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Factor Calculation For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculating ph for titration solutions: our titration calculator will help you never have to ask how do i calculate titrations?. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong.. Factor Calculation For Titration.