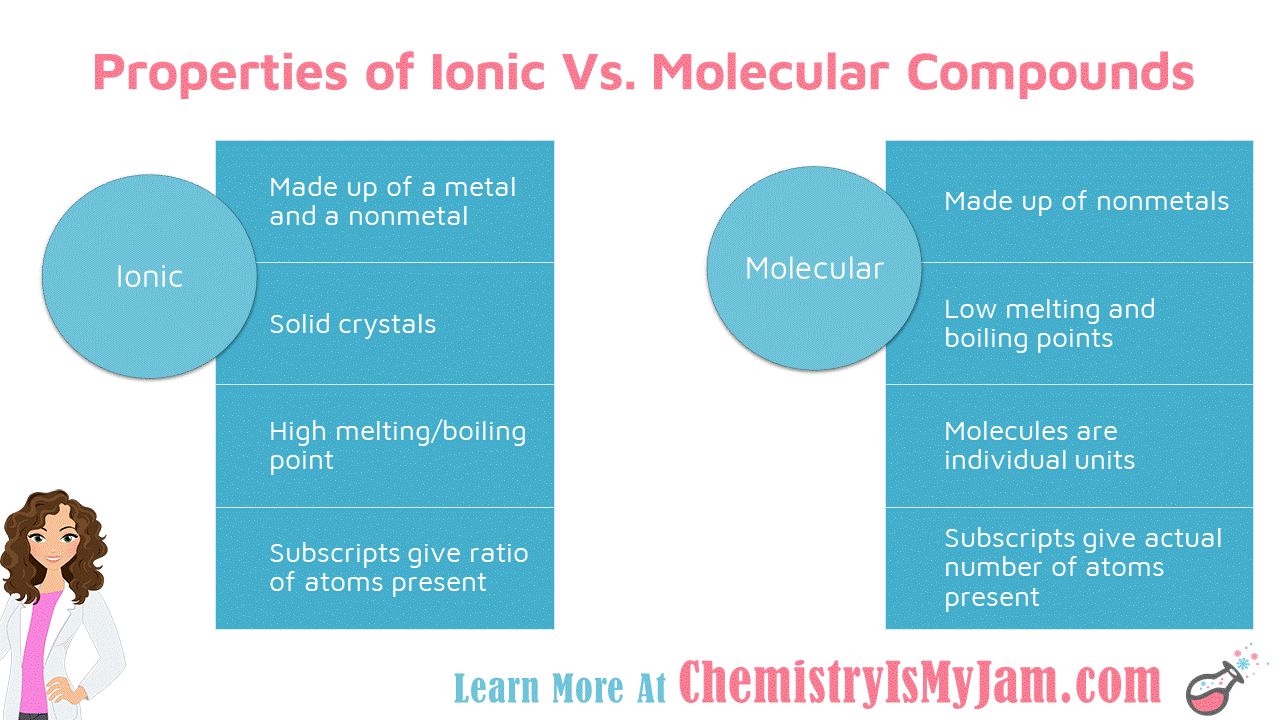
What Are Ionic And Molecular Compounds Give Examples . (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the periodic table. Updated on september 05, 2019. — ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. the difference between ionic and molecular compounds is that ionic compounds are composed of positive and negative ions. Ionic compounds are pure substances consisting of chemically. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. define ionic and molecular (covalent) compounds. — anne marie helmenstine, ph.d. Ionic bonds occur between two. Here are examples of ionic bonds and ionic. What is an ionic compound?
from chemistryismyjam.com
Electrostatic forces of attraction between the oppositely charged ions of an ionic compound. a compound that contains ions and is held together by ionic bonds is called an ionic compound. define ionic and molecular (covalent) compounds. (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. — anne marie helmenstine, ph.d. — ionic compounds. The periodic table can help us. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic compounds consist of atoms joined together by ionic bonds. define ionic and molecular (covalent) compounds.
Chemical Bonding Chemistry Is My Jam!
What Are Ionic And Molecular Compounds Give Examples — there are many examples of ionic compounds in everyday life. Predict the type of compound formed from elements based on their. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. (b) compare the properties of. define ionic and molecular (covalent) compounds. To name an inorganic compound, we need to consider the answers to several questions. ionic compounds are formed when ionic bonds are formed between different elements through the transfer of. Ionic bonds occur between two. (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. Electrostatic forces of attraction between the oppositely charged ions of an ionic compound. Atoms that gain or lose electrons are called ions, ions may have a. The periodic table can help us. Here are examples of ionic bonds and ionic.
From www.madebyteachers.com
Ionic & Molecular Compounds (Notes and Practice) Made By Teachers What Are Ionic And Molecular Compounds Give Examples — there are many examples of ionic compounds in everyday life. molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. Ionic compounds consist of atoms joined together by ionic bonds. What is an ionic compound? Electrostatic forces of attraction between the oppositely charged. What Are Ionic And Molecular Compounds Give Examples.
From learningschoolns1i1zoiu.z22.web.core.windows.net
Nomenclature Of Chemical Compounds What Are Ionic And Molecular Compounds Give Examples — a compound that contains ions and is held together by ionic bonds is called an ionic compound. — ionic compounds are composed of cations and anions that are strongly attracted to each other. Ionic compounds consist of atoms joined together by ionic bonds. Predict the type of compound formed from elements based on their location within the. What Are Ionic And Molecular Compounds Give Examples.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? What Are Ionic And Molecular Compounds Give Examples (b) compare the properties of. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Predict the type of compound formed from elements based on their. Ionic compounds are pure substances consisting of chemically. Atoms that gain or lose electrons are called ions, ions may have a. Predict the type of compound formed from elements based. What Are Ionic And Molecular Compounds Give Examples.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii What Are Ionic And Molecular Compounds Give Examples — ionic compounds. What is an ionic compound? (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. — ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. define ionic and molecular (covalent) compounds. — there are many examples of ionic compounds in everyday life.. What Are Ionic And Molecular Compounds Give Examples.
From en.wikipedia.org
Ionic bonding Wikipedia What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. — ionic compounds. To name an inorganic compound, we need to consider the answers to several questions. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein. What Are Ionic And Molecular Compounds Give Examples.
From www.youtube.com
Difference between Ionic Compounds and Molecular Compound YouTube What Are Ionic And Molecular Compounds Give Examples The periodic table can help us. define ionic and molecular (covalent) compounds. Ionic compounds are pure substances consisting of chemically. To name an inorganic compound, we need to consider the answers to several questions. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. a compound that contains ions. What Are Ionic And Molecular Compounds Give Examples.
From utaheducationfacts.com
How To Write Molecular What Are Ionic And Molecular Compounds Give Examples — a compound that contains ions and is held together by ionic bonds is called an ionic compound. define ionic and molecular (covalent) compounds. Atoms that gain or lose electrons are called ions, ions may have a. Predict the type of compound formed from elements based on their location within the. ionic compounds include salts, oxides, hydroxides,. What Are Ionic And Molecular Compounds Give Examples.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. ionic compounds are formed when ionic bonds are formed between different elements through the transfer of. define ionic and molecular (covalent) compounds. The periodic table can help us. define ionic and molecular (covalent) compounds. (b) compare the properties of. the difference between ionic and molecular. What Are Ionic And Molecular Compounds Give Examples.
From www.expii.com
Ionic Bond — Formation & Compounds Expii What Are Ionic And Molecular Compounds Give Examples — anne marie helmenstine, ph.d. Atoms that gain or lose electrons are called ions, ions may have a. Ionic compounds are pure substances consisting of chemically. define ionic and molecular (covalent) compounds. (b) compare the properties of. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic compounds consist of atoms joined together. What Are Ionic And Molecular Compounds Give Examples.
From pediaa.com
Difference Between Ionic and Molecular Compounds What Are Ionic And Molecular Compounds Give Examples define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. Ionic bonds occur between two. Ionic compounds consist of atoms joined together by ionic bonds. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. — there are many examples of ionic compounds in everyday life.. What Are Ionic And Molecular Compounds Give Examples.
From www.youtube.com
Naming Ionic Compounds, a tutorial Crash Chemistry Academy YouTube What Are Ionic And Molecular Compounds Give Examples ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Ionic compounds consist of atoms joined together by ionic bonds. define ionic and molecular (covalent) compounds. To name an inorganic compound, we need to consider the answers to several questions. Electrostatic forces of attraction between. What Are Ionic And Molecular Compounds Give Examples.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry What Are Ionic And Molecular Compounds Give Examples molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. The periodic table can help us. (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. — ionic compounds are composed of cations and anions that are strongly attracted. What Are Ionic And Molecular Compounds Give Examples.
From www.slideserve.com
PPT IONIC COMPOUNDS PowerPoint Presentation, free download ID907550 What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. — anne marie helmenstine, ph.d. Predict the type of compound formed from elements based on their location within the periodic table. — ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. Atoms that gain or lose electrons are called ions, ions. What Are Ionic And Molecular Compounds Give Examples.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. Atoms that gain or lose electrons are called ions, ions may have a. molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. — there are many examples of ionic compounds in everyday. What Are Ionic And Molecular Compounds Give Examples.
From www.youtube.com
Ionic Compounds vs. Covalent Molecules. (Chemistry Ch. 5, Part 5) YouTube What Are Ionic And Molecular Compounds Give Examples (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic compound, any of a. What Are Ionic And Molecular Compounds Give Examples.
From www.sliderbase.com
Naming Ionic Compounds What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. define ionic and molecular (covalent) compounds. — there are many examples of ionic compounds in everyday life. The periodic table can help us. a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic compounds are formed when. What Are Ionic And Molecular Compounds Give Examples.
From cabinet.matttroy.net
Is Table Salt A Molecular Compound Matttroy What Are Ionic And Molecular Compounds Give Examples Ionic compounds are pure substances consisting of chemically. Predict the type of compound formed from elements based on their location within the. a compound that contains ions and is held together by ionic bonds is called an ionic compound. To name an inorganic compound, we need to consider the answers to several questions. — ionic compounds are composed. What Are Ionic And Molecular Compounds Give Examples.
From sciencenotes.org
Covalent Compounds Examples and Properties What Are Ionic And Molecular Compounds Give Examples a compound that contains ions and is held together by ionic bonds is called an ionic compound. Predict the type of compound formed from elements based on their. the difference between ionic and molecular compounds is that ionic compounds are composed of positive and negative ions. To name an inorganic compound, we need to consider the answers to. What Are Ionic And Molecular Compounds Give Examples.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry What Are Ionic And Molecular Compounds Give Examples Electrostatic forces of attraction between the oppositely charged ions of an ionic compound. Predict the type of compound formed from elements based on their location within the. — ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. Predict the type of compound formed from elements based on their. The periodic table can help. What Are Ionic And Molecular Compounds Give Examples.
From ar.inspiredpencil.com
Ionic Compounds Chart What Are Ionic And Molecular Compounds Give Examples To name an inorganic compound, we need to consider the answers to several questions. The periodic table can help us. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. (b) compare the properties of. Ionic compounds consist of atoms joined together by ionic bonds. Predict the type of compound formed. What Are Ionic And Molecular Compounds Give Examples.
From astonishingceiyrs.blogspot.com
Ionic Bond Properties astonishingceiyrs What Are Ionic And Molecular Compounds Give Examples Predict the type of compound formed from elements based on their. — there are many examples of ionic compounds in everyday life. — ionic compounds. Predict the type of compound formed from elements based on their location within the. Predict the type of compound formed from elements based on their location within the periodic table. define ionic. What Are Ionic And Molecular Compounds Give Examples.
From www.teachoo.com
Molecules and Compounds Definition, Differenences [in Table Form] What Are Ionic And Molecular Compounds Give Examples — a compound that contains ions and is held together by ionic bonds is called an ionic compound. a compound that contains ions and is held together by ionic bonds is called an ionic compound. Atoms that gain or lose electrons are called ions, ions may have a. ionic compounds are formed when ionic bonds are formed. What Are Ionic And Molecular Compounds Give Examples.
From www.youtube.com
Examples of Ionic Compoiunds YouTube What Are Ionic And Molecular Compounds Give Examples Ionic compounds are pure substances consisting of chemically. Here are examples of ionic bonds and ionic. Ionic bonds occur between two. Predict the type of compound formed from elements based on their. Predict the type of compound formed from elements based on their location within the periodic table. Atoms that gain or lose electrons are called ions, ions may have. What Are Ionic And Molecular Compounds Give Examples.
From socratic.org
What is an example of ionic bonds practice problem? Socratic What Are Ionic And Molecular Compounds Give Examples Updated on september 05, 2019. — anne marie helmenstine, ph.d. Ionic bonds occur between two. define ionic and molecular (covalent) compounds. define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. What is an ionic compound? compounds that consist of ions are known as ionic atoms.. What Are Ionic And Molecular Compounds Give Examples.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation, free What Are Ionic And Molecular Compounds Give Examples Here are examples of ionic bonds and ionic. — there are many examples of ionic compounds in everyday life. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. a compound that contains ions and. What Are Ionic And Molecular Compounds Give Examples.
From www.youtube.com
Writing Formulas For Ionic Compounds YouTube What Are Ionic And Molecular Compounds Give Examples To name an inorganic compound, we need to consider the answers to several questions. Here are examples of ionic bonds and ionic. — anne marie helmenstine, ph.d. molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. ionic compounds include salts, oxides, hydroxides,. What Are Ionic And Molecular Compounds Give Examples.
From circuitlibraryspaes.z13.web.core.windows.net
Diagram Of Compound What Are Ionic And Molecular Compounds Give Examples — ionic compounds are composed of cations and anions that are strongly attracted to each other. define ionic and molecular (covalent) compounds. compounds that consist of ions are known as ionic atoms. — a compound that contains ions and is held together by ionic bonds is called an ionic compound. — there are many examples. What Are Ionic And Molecular Compounds Give Examples.
From chemistryismyjam.com
Chemical Bonding Chemistry Is My Jam! What Are Ionic And Molecular Compounds Give Examples ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. compounds that consist of ions are known as ionic atoms. Updated on september 05, 2019. To name an inorganic compound, we need to consider the answers to several questions. Predict the type of compound formed from elements based on their. — anne marie helmenstine,. What Are Ionic And Molecular Compounds Give Examples.
From www.compoundworksheets.com
Naming Ionic Compounds Worksheet Pdf What Are Ionic And Molecular Compounds Give Examples — ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Ionic compounds are pure substances consisting of chemically. — there are many examples of ionic compounds in everyday. What Are Ionic And Molecular Compounds Give Examples.
From chem.libretexts.org
6.2 Comparing Ionic and Molecular Substances Chemistry LibreTexts What Are Ionic And Molecular Compounds Give Examples define ionic and molecular (covalent) compounds. molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element. Predict the type of compound formed from elements based on their location within the periodic table. define ionic and molecular (covalent) compounds. The periodic table can help. What Are Ionic And Molecular Compounds Give Examples.
From chem.libretexts.org
Chapter 4.4 Naming Ionic Compounds Chemistry LibreTexts What Are Ionic And Molecular Compounds Give Examples ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. The periodic table can help us. Ionic compounds are pure substances consisting of chemically. To name an inorganic compound, we need to consider the answers to several questions. Here are examples of ionic bonds and ionic. define ionic and molecular (covalent) compounds. define ionic. What Are Ionic And Molecular Compounds Give Examples.
From studylib.net
Ionic Compounds Naming What Are Ionic And Molecular Compounds Give Examples To name an inorganic compound, we need to consider the answers to several questions. — ionic compounds are composed of cations and anions that are strongly attracted to each other. — there are many examples of ionic compounds in everyday life. ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Electrostatic forces of. What Are Ionic And Molecular Compounds Give Examples.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry What Are Ionic And Molecular Compounds Give Examples a compound that contains ions and is held together by ionic bonds is called an ionic compound. — anne marie helmenstine, ph.d. — ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. To name an inorganic compound, we need to consider the answers to several questions. molecular compounds can form. What Are Ionic And Molecular Compounds Give Examples.
From saylordotorg.github.io
Chemical Compounds What Are Ionic And Molecular Compounds Give Examples define ionic and molecular (covalent) compounds. a compound that contains ions and is held together by ionic bonds is called an ionic compound. Predict the type of compound formed from elements based on their location within the periodic table. Ionic bonds occur between two. define ionic and molecular (covalent) compounds. — ionic compounds are made of. What Are Ionic And Molecular Compounds Give Examples.
From breadandhearth.com
Formulas And Names Of Compounds Worksheet Breadandhearth What Are Ionic And Molecular Compounds Give Examples — ionic compounds. Predict the type of compound formed from elements based on their. a compound that contains ions and is held together by ionic bonds is called an ionic compound. (a) giving one example each, state what are (i) ionic compounds, and (ii) covalent compounds. define ionic and molecular (covalent) compounds. Electrostatic forces of attraction between. What Are Ionic And Molecular Compounds Give Examples.