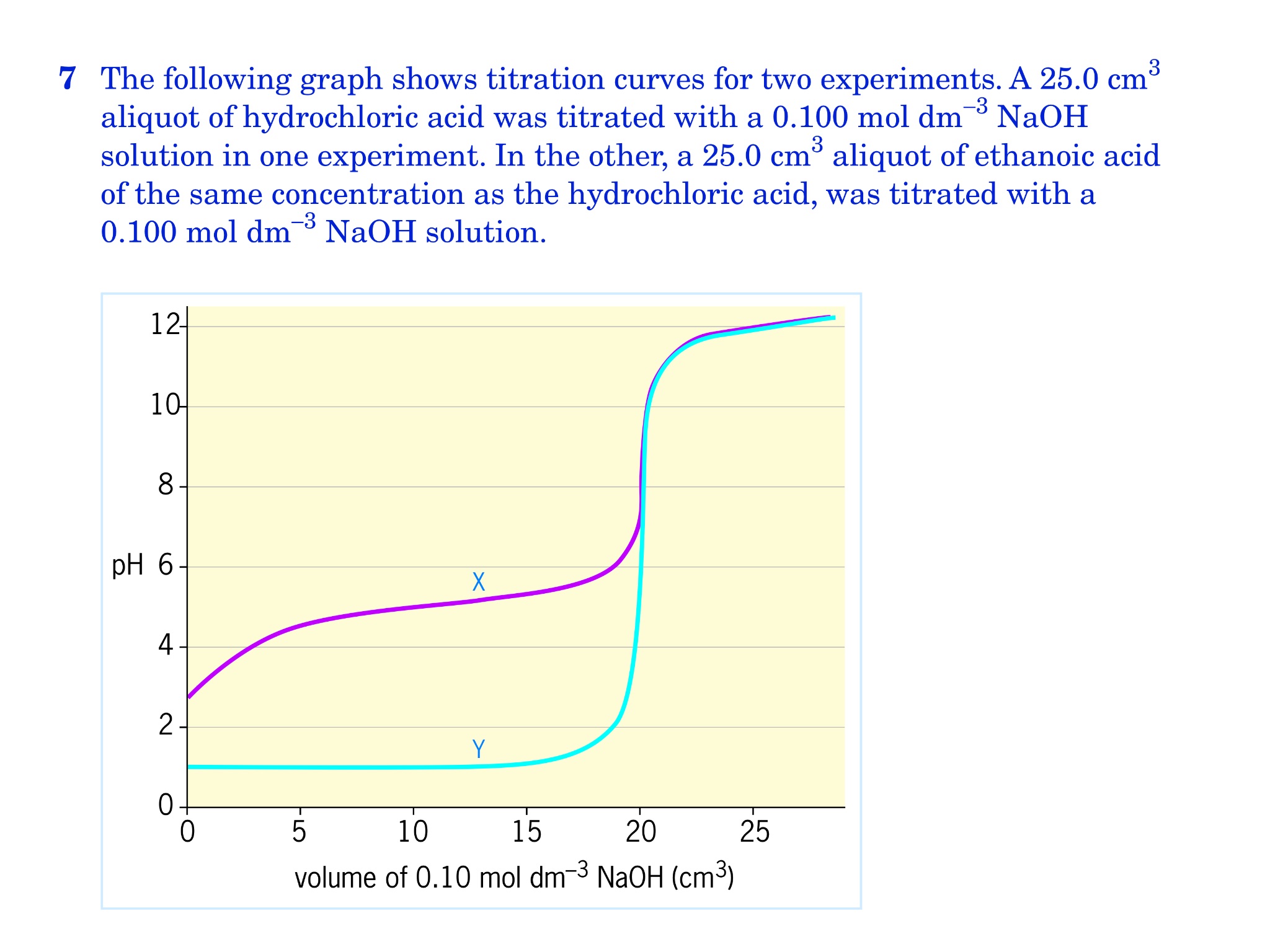
How To Calculate Concentration Of Weak Acid From Titration Curve . Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. Determine the k a or k b value for a weak acid or weak base. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Initially, the ph of a weak acid like. In each case, you start with 25 cm 3 of one of. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. 🧠 for all my science videos and resources:
from socratic.org
Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. The reaction of the weak. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine the k a or k b value for a weak acid or weak base. Initially, the ph of a weak acid like. In each case, you start with 25 cm 3 of one of. 🧠 for all my science videos and resources:
How to calculate the concentration of the acid solutions? Socratic
How To Calculate Concentration Of Weak Acid From Titration Curve Initially, the ph of a weak acid like. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The reaction of the weak. Initially, the ph of a weak acid like. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. Determine the k a or k b value for a weak acid or weak base. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 🧠 for all my science videos and resources: In each case, you start with 25 cm 3 of one of.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 How To Calculate Concentration Of Weak Acid From Titration Curve In each case, you start with 25 cm 3 of one of. 🧠 for all my science videos and resources: The reaction of the weak. Initially, the ph of a weak acid like. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. In titrating a. How To Calculate Concentration Of Weak Acid From Titration Curve.
From webmis.highland.cc.il.us
AcidBase Titrations How To Calculate Concentration Of Weak Acid From Titration Curve In each case, you start with 25 cm 3 of one of. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Chemists often calculate the acidity of the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A titration. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How To Calculate Concentration Of Weak Acid From Titration Curve In each case, you start with 25 cm 3 of one of. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. 🧠 for all my science videos and. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. In each case, you start with 25 cm 3 of one of. Initially, the ph of a weak acid like. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine the k a or k b value for a weak. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts How To Calculate Concentration Of Weak Acid From Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 🧠 for all my science videos and resources: In each case, you start with 25 cm 3 of one of. Determine ph and concentrations of chemical species at any point in the titration of a weak. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The reaction of the weak. In each case, you start with 25 cm 3 of one of. 🧠 for all my science videos and resources: Chemists often calculate the acidity of. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. 🧠 for all my science videos and resources: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In each case, you. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.writework.com
Titration of amino acids WriteWork How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. Determine the k a or k b value for a weak acid or weak base. The titration of a weak acid with a strong base involves the direct transfer of protons from. How To Calculate Concentration Of Weak Acid From Titration Curve.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts How To Calculate Concentration Of Weak Acid From Titration Curve In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. In each case, you start with 25 cm 3 of one of. Chemists often calculate the acidity of the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From studylib.net
Titration Curve weak base with strong acid START How To Calculate Concentration Of Weak Acid From Titration Curve In each case, you start with 25 cm 3 of one of. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Initially, the ph of a weak acid like. The titration of a weak acid with a strong base involves. How To Calculate Concentration Of Weak Acid From Titration Curve.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves How To Calculate Concentration Of Weak Acid From Titration Curve A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists often calculate the acidity of the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers How To Calculate Concentration Of Weak Acid From Titration Curve Determine the k a or k b value for a weak acid or weak base. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak acid like. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid. How To Calculate Concentration Of Weak Acid From Titration Curve.
From exykmbsij.blob.core.windows.net
Buffer Zone On Titration Curve at James Davis blog How To Calculate Concentration Of Weak Acid From Titration Curve In each case, you start with 25 cm 3 of one of. Initially, the ph of a weak acid like. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. 🧠 for all my science videos and resources: A titration curve. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. The reaction of the weak. Initially, the ph of a weak acid like. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. In each case, you start with 25. How To Calculate Concentration Of Weak Acid From Titration Curve.
From mungfali.com
Titration Curve Labeled How To Calculate Concentration Of Weak Acid From Titration Curve In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak acid like. Determine the k a or k b value for a weak acid or weak base. The reaction of the weak. A titration curve is a plot of the concentration of the analyte at a given. How To Calculate Concentration Of Weak Acid From Titration Curve.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How To Calculate Concentration Of Weak Acid From Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Initially, the ph of a weak acid like. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. The reaction of the weak. Chemists often calculate the acidity of. How To Calculate Concentration Of Weak Acid From Titration Curve.
From philschatz.com
AcidBase Titrations · Chemistry How To Calculate Concentration Of Weak Acid From Titration Curve Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. 🧠 for all my science videos and resources: Initially, the ph of a weak acid like. Determine the k a or k b value for a weak acid or weak base.. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.slideserve.com
PPT Experiment 23 C Titration Curves for Strong Acid and Weak Acids How To Calculate Concentration Of Weak Acid From Titration Curve Initially, the ph of a weak acid like. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. Determine the k a or k b value for a weak acid or weak base. Determine ph and concentrations of chemical species at any point in the titration of. How To Calculate Concentration Of Weak Acid From Titration Curve.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog How To Calculate Concentration Of Weak Acid From Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 🧠 for all my science videos and resources: Initially, the ph of a weak acid like. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Chemists often calculate. How To Calculate Concentration Of Weak Acid From Titration Curve.
From mungfali.com
Acid Base Titration Calculation How To Calculate Concentration Of Weak Acid From Titration Curve Initially, the ph of a weak acid like. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. 🧠 for all my science. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. 🧠 for all my science videos and resources: Initially, the ph of a weak acid like. The titration of a weak acid with a strong base involves the direct transfer of. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A How To Calculate Concentration Of Weak Acid From Titration Curve Initially, the ph of a weak acid like. The reaction of the weak. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. A titration curve is a plot of. How To Calculate Concentration Of Weak Acid From Titration Curve.
From courses.lumenlearning.com
15.2 AcidBase Titrations Chemistry How To Calculate Concentration Of Weak Acid From Titration Curve Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a. How To Calculate Concentration Of Weak Acid From Titration Curve.
From yojkglpsgc.blogspot.com
How To Calculate Initial Concentration From Titration Curve Volume of How To Calculate Concentration Of Weak Acid From Titration Curve 🧠 for all my science videos and resources: The reaction of the weak. Initially, the ph of a weak acid like. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
How to Calculate the Hydrogen Ion Concentration of a Weak Acid Solution How To Calculate Concentration Of Weak Acid From Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In each case, you start with 25 cm 3 of one of. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Calculate Concentration Of Weak Acid From Titration Curve In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Chemists often. How To Calculate Concentration Of Weak Acid From Titration Curve.
From lessonlibillocution.z22.web.core.windows.net
How To Calculate Acid Base How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. 🧠 for all my science videos and resources: Determine the k a or k b value for a weak acid or weak base. In each case, you start with 25 cm 3 of one of. Initially, the ph of a weak acid like. Determine ph and concentrations of chemical species at any point in the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Concentration Of Weak Acid From Titration Curve The reaction of the weak. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In each case, you start with 25 cm 3 of one of. A titration curve is a plot of the concentration of the analyte at a given point in the experiment. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Concentration Of Weak Acid From Titration Curve A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. 🧠 for all my science videos and resources: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the. How To Calculate Concentration Of Weak Acid From Titration Curve.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube How To Calculate Concentration Of Weak Acid From Titration Curve Initially, the ph of a weak acid like. The reaction of the weak. Determine the k a or k b value for a weak acid or weak base. In each case, you start with 25 cm 3 of one of. Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge. How To Calculate Concentration Of Weak Acid From Titration Curve.
From srkwxfutjcwku.blogspot.com
How To Find Initial Concentration From Titration Curve The initial How To Calculate Concentration Of Weak Acid From Titration Curve In titrating a weak acid with a strong base, different calculation methods are applied at various stages. 🧠 for all my science videos and resources: Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The reaction of the weak. Chemists. How To Calculate Concentration Of Weak Acid From Titration Curve.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog How To Calculate Concentration Of Weak Acid From Titration Curve A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. Initially, the ph of a weak acid. How To Calculate Concentration Of Weak Acid From Titration Curve.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate Concentration Of Weak Acid From Titration Curve Chemists often calculate the acidity of the analyte at some point between the initial and the equivalence points to gauge the precise formation. In each case, you start with 25 cm 3 of one of. A titration curve is a plot of the concentration of the analyte at a given point in the experiment (usually ph in an acid. The. How To Calculate Concentration Of Weak Acid From Titration Curve.