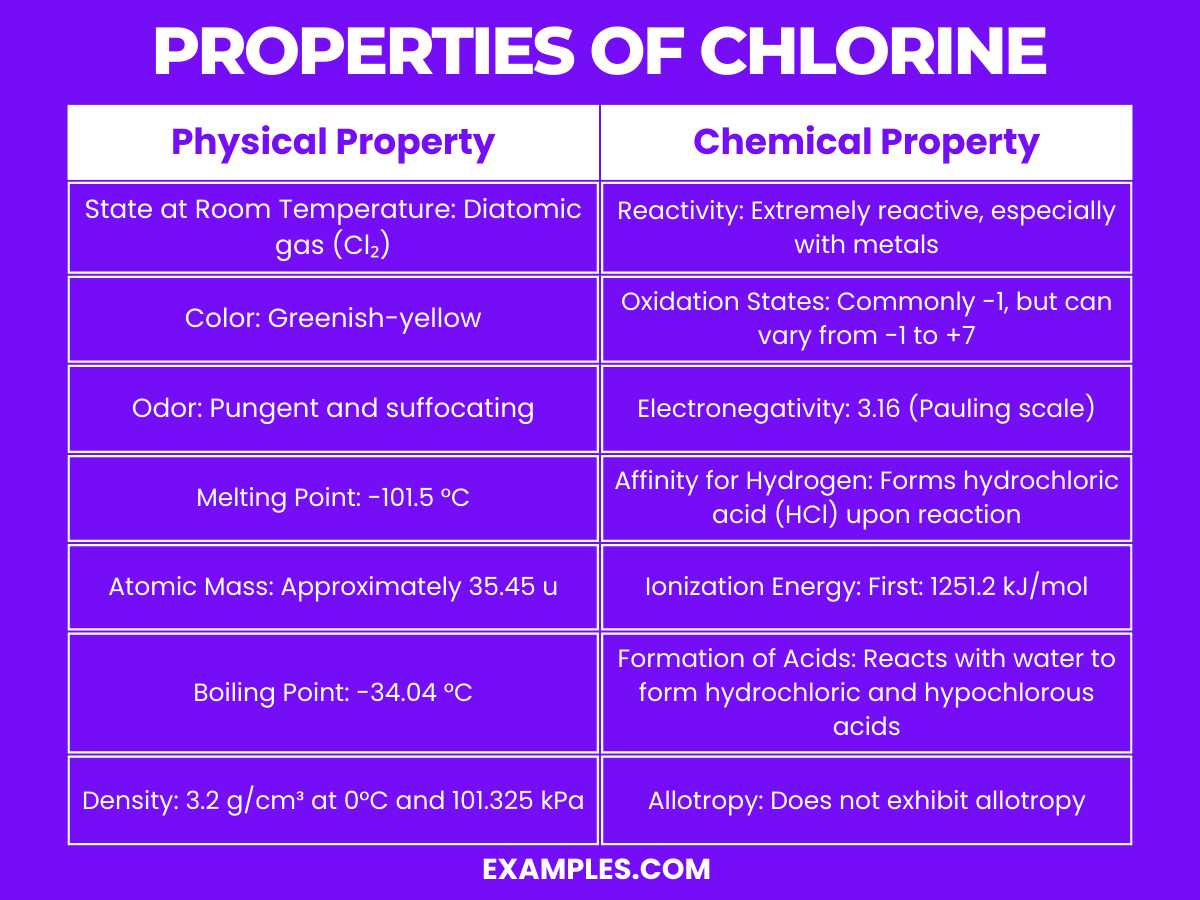
Properties Of Chlorine And Iodine . Molecules close molecule a collection of two or. It has a strong oxidising ability. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The three common group 7 elements are chlorine, bromine and iodine. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. The group 7 elements are also known as the halogens. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. The halogens are located on the left of the noble gases on the periodic table. The word ‘halogen’ means 'salt former'.
from www.examples.com
The group 7 elements are also known as the halogens. The halogens are located on the left of the noble gases on the periodic table. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. Molecules close molecule a collection of two or. It has a strong oxidising ability. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and iodine. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules.
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds
Properties Of Chlorine And Iodine Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Molecules close molecule a collection of two or. The word ‘halogen’ means 'salt former'. The halogens are located on the left of the noble gases on the periodic table. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. It has a strong oxidising ability. The group 7 elements are also known as the halogens. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and iodine.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table Properties Of Chlorine And Iodine For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. It has a strong oxidising ability. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine,. Properties Of Chlorine And Iodine.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Properties Of Chlorine And Iodine The group 7 elements are also known as the halogens. The word ‘halogen’ means 'salt former'. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. The halogens are located on the left of the noble gases on the periodic table. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and. Properties Of Chlorine And Iodine.
From www.youtube.com
ICl Lewis Structure How to Draw the Lewis Structure for the Iodine Properties Of Chlorine And Iodine Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. The three common group 7 elements are chlorine, bromine and iodine. The halogens are located on the left of the noble gases on the periodic table. It has a strong oxidising ability. The word ‘halogen’ means 'salt. Properties Of Chlorine And Iodine.
From www.youtube.com
Iodine + chlorine YouTube Properties Of Chlorine And Iodine Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Molecules close molecule a collection of two or. The halogens are located on the left of the noble gases on the periodic table. The group 7 elements are also known as the halogens. Chlorine (atomic number 17) and iodine (atomic number. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1878282 Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. The group 7 elements are also known as the halogens. It has a strong oxidising ability. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase. Properties Of Chlorine And Iodine.
From www.scribd.com
Group Properties PDF Iodine Chlorine Properties Of Chlorine And Iodine The word ‘halogen’ means 'salt former'. The halogens are located on the left of the noble gases on the periodic table. The three common group 7 elements are chlorine, bromine and iodine. Molecules close molecule a collection of two or. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. For nonpolar molecules,. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142 Properties Of Chlorine And Iodine Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. The group 7 elements are also known as the halogens. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and. Properties Of Chlorine And Iodine.
From www.examples.com
Iodine (I) Definition, Preparation, Properties, Uses, Compounds Properties Of Chlorine And Iodine It has a strong oxidising ability. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. The group 7 elements are also known as the halogens. Molecules close molecule a collection of two or. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chlorine ( Cl ) PowerPoint Presentation, free download ID2276942 Properties Of Chlorine And Iodine The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and iodine. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free Properties Of Chlorine And Iodine Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Molecules close molecule. Properties Of Chlorine And Iodine.
From www.youtube.com
Iodine + Sodium Chloride YouTube Properties Of Chlorine And Iodine Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. The halogens are located on the left of the noble gases on the periodic table. The word ‘halogen’ means 'salt former'. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular. Properties Of Chlorine And Iodine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Properties Of Chlorine And Iodine Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. It has a strong oxidising ability. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens. The halogens. Properties Of Chlorine And Iodine.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Properties Of Chlorine And Iodine The word ‘halogen’ means 'salt former'. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. The group 7 elements are also known as the halogens. The halogens are located on. Properties Of Chlorine And Iodine.
From elchoroukhost.net
Chlorine Periodic Table Properties Elcho Table Properties Of Chlorine And Iodine It has a strong oxidising ability. Molecules close molecule a collection of two or. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. The seven diatomic elements are iodine, bromine, chlorine,. Properties Of Chlorine And Iodine.
From dkhfxaogeco.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Benjamin Lakin blog Properties Of Chlorine And Iodine This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. The three common group 7 elements are chlorine, bromine and iodine. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The word ‘halogen’ means 'salt former'. For nonpolar molecules,. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chapter 4 Formation of Compounds PowerPoint Presentation, free Properties Of Chlorine And Iodine The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. It has a strong oxidising ability. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. The group 7 elements are also. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID8800875 Properties Of Chlorine And Iodine The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. The three common group 7 elements are chlorine, bromine and iodine. The halogens are. Properties Of Chlorine And Iodine.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. The group 7 elements are also known as the halogens. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Descending the. Properties Of Chlorine And Iodine.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. It has a strong oxidising ability. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and. Properties Of Chlorine And Iodine.
From utedzz.blogspot.com
Chlorine Bromine Periodic Table Periodic Table Timeline Properties Of Chlorine And Iodine The three common group 7 elements are chlorine, bromine and iodine. It has a strong oxidising ability. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Descending the group, fluorine and chlorine are gases, bromine is a. Properties Of Chlorine And Iodine.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The group 7 elements are also known as the halogens. For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. It has a strong oxidising ability. Chlorine. Properties Of Chlorine And Iodine.
From material-properties.org
Chlorine and Iodine Comparison Properties Material Properties Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The group 7 elements are also known as the halogens. Descending the. Properties Of Chlorine And Iodine.
From sciencechemistri.blogspot.com
Science Chemistry Properties of Chlorine, Bromine, Iodine and Astatine Properties Of Chlorine And Iodine The word ‘halogen’ means 'salt former'. Chlorine (atomic number 17) and iodine (atomic number 53) have different physical states at room temperature due to the increase in molecular size. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Molecules close molecule a collection of two or. It has a strong. Properties Of Chlorine And Iodine.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Properties Of Chlorine And Iodine The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. For nonpolar molecules, the farther you go. Properties Of Chlorine And Iodine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Properties Of Chlorine And Iodine The halogens are located on the left of the noble gases on the periodic table. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The word ‘halogen’ means 'salt former'. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. Chlorine (atomic number 17) and iodine (atomic. Properties Of Chlorine And Iodine.
From www.sciencephoto.com
Chlorine displacing iodine Stock Image A500/0386 Science Photo Properties Of Chlorine And Iodine Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The halogens are located on the left of the noble gases on the periodic table. Molecules close molecule a collection of two or. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements. Properties Of Chlorine And Iodine.
From www.shutterstock.com
Iodine Trichloride Icl3 Cl3i Interhalogen Compound ilustración de Properties Of Chlorine And Iodine The halogens are located on the left of the noble gases on the periodic table. The three common group 7 elements are chlorine, bromine and iodine. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two. Properties Of Chlorine And Iodine.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Properties Of Chlorine And Iodine Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Molecules close molecule a collection of two or. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable. Properties Of Chlorine And Iodine.
From www.vedantu.com
Chlorine Learn Definition, Properties and Facts Properties Of Chlorine And Iodine Molecules close molecule a collection of two or. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. The halogens are located on the left of the noble gases on the periodic table. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common. Properties Of Chlorine And Iodine.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids Properties Of Chlorine And Iodine The word ‘halogen’ means 'salt former'. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Molecules close molecule a collection of two or. The three common group 7 elements are chlorine, bromine and iodine. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements. Properties Of Chlorine And Iodine.
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica Properties Of Chlorine And Iodine It has a strong oxidising ability. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Chlorine (atomic. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Chlorine PowerPoint Presentation, free download ID2277012 Properties Of Chlorine And Iodine The group 7 elements are also known as the halogens. The seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. Molecules close molecule a collection of two or. The three common group 7 elements are chlorine, bromine and iodine. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2. Properties Of Chlorine And Iodine.
From studylib.net
Group 7 Elements Properties Of Chlorine And Iodine For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. It has a strong oxidising ability. The group 7 elements are also known as the halogens. Iodine is soluble in chloroform, carbon tetrachloride, carbon disulfide, and many hydrocarbons, giving violet solutions of i 2 molecules. The word ‘halogen’ means 'salt former'. Descending the group,. Properties Of Chlorine And Iodine.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID2844469 Properties Of Chlorine And Iodine For nonpolar molecules, the farther you go down the group, the stronger the london dispersion forces. The halogens are located on the left of the noble gases on the periodic table. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Chlorine (atomic number 17) and iodine (atomic number. Properties Of Chlorine And Iodine.
From www.slideshare.net
Lecture 6.1 The Periodic Table Properties Of Chlorine And Iodine It has a strong oxidising ability. This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Molecules close molecule a collection of two or. The three common group 7 elements are chlorine,. Properties Of Chlorine And Iodine.