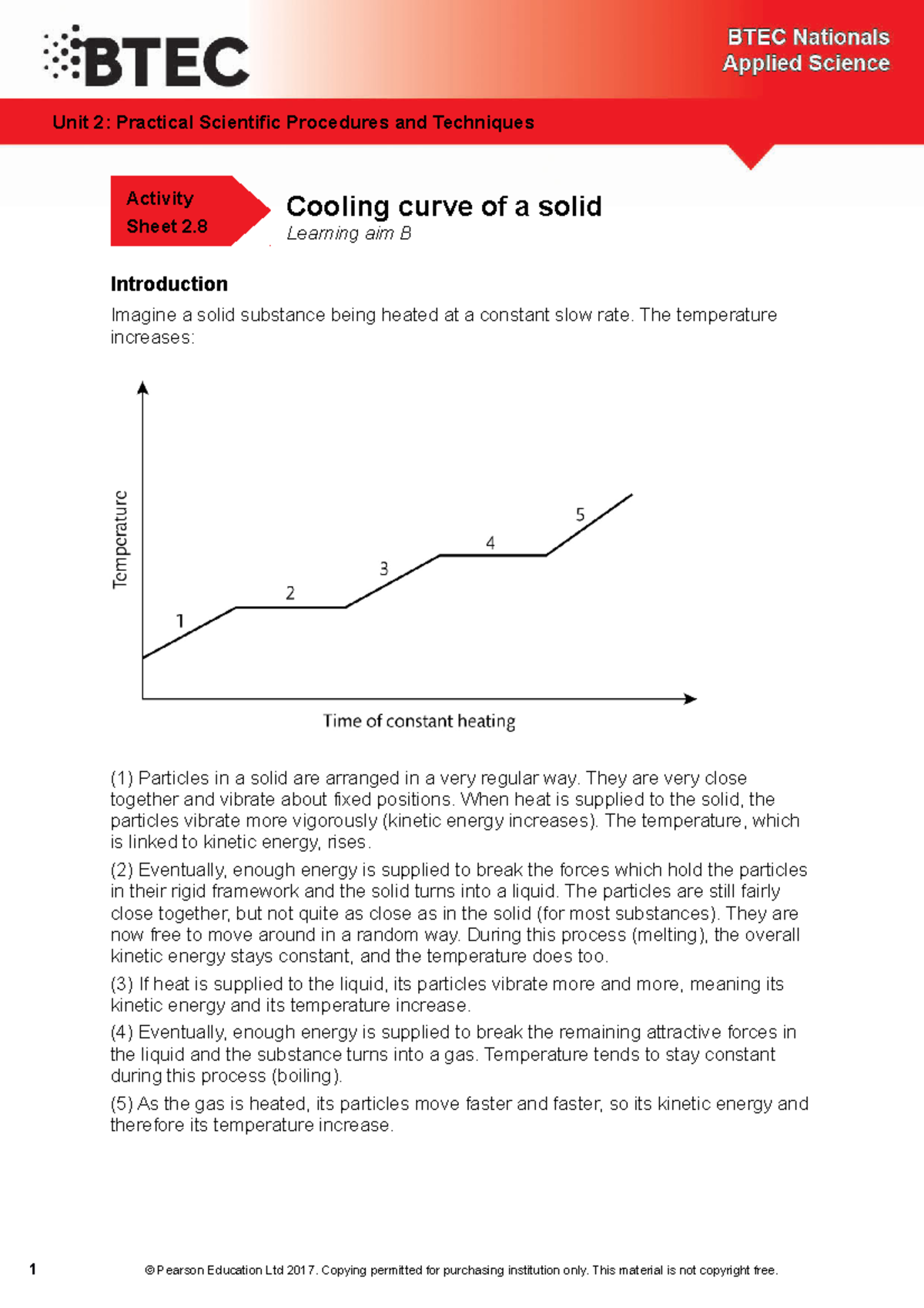
Solid Heating And Cooling Curve . For heating curves, we start with a solid and add heat energy. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Heating curves relate temperature changes to phase transitions. Evaporation occurs when a liquid turns to a gas. An experiment can be planned to measure the temperature change through continuous heating of a substance. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. For cooling curves, we start with the gas phase and.
from www.studocu.com
The curve is divided into distinct segments, each corresponding to a specific phase of the substance. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. For cooling curves, we start with the gas phase and. For heating curves, we start with a solid and add heat energy. An experiment can be planned to measure the temperature change through continuous heating of a substance. Heating curves relate temperature changes to phase transitions. Evaporation occurs when a liquid turns to a gas.
Heating and cooling curve worksheet Cooling curve of a solid Learning
Solid Heating And Cooling Curve All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Evaporation occurs when a liquid turns to a gas. An experiment can be planned to measure the temperature change through continuous heating of a substance. For cooling curves, we start with the gas phase and. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. For heating curves, we start with a solid and add heat energy. Heating curves relate temperature changes to phase transitions. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below.
From slideplayer.com
Heating and Cooling Curves ppt download Solid Heating And Cooling Curve For cooling curves, we start with the gas phase and. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Evaporation occurs when a liquid turns to a gas. Heating curves relate. Solid Heating And Cooling Curve.
From ch301.cm.utexas.edu
heating curve Solid Heating And Cooling Curve Heating curves relate temperature changes to phase transitions. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. An experiment can be planned to measure the temperature change through continuous heating of a substance. Evaporation occurs when a liquid turns to a gas. Heat, cool and compress atoms and molecules and watch as. Solid Heating And Cooling Curve.
From www.worldwisetutoring.com
Heating and Cooling Curves Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. Heating curves relate temperature changes to phase transitions. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. For heating curves, we start with a solid and add heat energy. The curve is divided into distinct segments, each corresponding. Solid Heating And Cooling Curve.
From byjus.com
Explain the cooling curve in crystalline and amorphous solids. Solid Heating And Cooling Curve An experiment can be planned to measure the temperature change through continuous heating of a substance. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. For cooling curves, we start with the gas phase and. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.. Solid Heating And Cooling Curve.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Solid Heating And Cooling Curve Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heating curves relate temperature changes. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT States of Matter! PowerPoint Presentation, free download ID6851506 Solid Heating And Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. For cooling curves, we start with the gas phase and. Heating curves relate temperature changes to phase transitions. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. An experiment can be planned. Solid Heating And Cooling Curve.
From lessonschoolwinchell.z21.web.core.windows.net
Heat And Cooling Curve Solid Heating And Cooling Curve All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. An experiment can be planned to measure the temperature change through continuous heating of a substance. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Evaporation occurs. Solid Heating And Cooling Curve.
From www.youtube.com
How to Read a Cooling Curve YouTube Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. An experiment can be planned to measure the temperature change through continuous heating of a substance.. Solid Heating And Cooling Curve.
From www.studocu.com
Heating and cooling curve worksheet Cooling curve of a solid Learning Solid Heating And Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. An experiment can be planned to measure the temperature change through continuous heating of a substance. Heat, cool and compress atoms and molecules and. Solid Heating And Cooling Curve.
From www.youtube.com
COOLING CURVE CRYSTALLINE & AMORPHOUS SOLIDS POLYMER CHEMISTRY Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. For heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. An experiment can be planned to measure the temperature change through. Solid Heating And Cooling Curve.
From www.savemyexams.com
Heating & Cooling Graphs AQA GCSE Physics Revision Notes 2018 Solid Heating And Cooling Curve All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. For heating curves, we start with a solid and add heat energy. The curve is divided into distinct. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Solid Heating And Cooling Curve For heating curves, we start with a solid and add heat energy. Heating curves relate temperature changes to phase transitions. For cooling curves, we start with the gas phase and. Evaporation occurs when a liquid turns to a gas. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure. Solid Heating And Cooling Curve.
From answerzoneschuster.z21.web.core.windows.net
Heat And Cooling Curve Solid Heating And Cooling Curve An experiment can be planned to measure the temperature change through continuous heating of a substance. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Evaporation occurs when a liquid turns to a gas. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Solid Heating And Cooling Curve For heating curves, we start with a solid and add heat energy. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. A superheated liquid, a liquid at a temperature and pressure at which it should be. Solid Heating And Cooling Curve.
From www.showme.com
Heating and Cooling Curves Explained Science, Heating Curve, Cooling Solid Heating And Cooling Curve Heating curves relate temperature changes to phase transitions. An experiment can be planned to measure the temperature change through continuous heating of a substance. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. For heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas. Solid Heating And Cooling Curve.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Solid Heating And Cooling Curve Heating curves relate temperature changes to phase transitions. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. An experiment can be planned to measure the temperature change through continuous heating of a substance. For heating curves, we start with a solid and add heat energy. Heat, cool. Solid Heating And Cooling Curve.
From www.researchgate.net
3) Cooling curve for a pure metal. Download Scientific Diagram Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. For heating curves, we start with a solid and add heat energy. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. An. Solid Heating And Cooling Curve.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Solid Heating And Cooling Curve All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. Heating curves relate temperature changes to phase transitions. Heat, cool and compress atoms and molecules and watch as they change between. Solid Heating And Cooling Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Solid Heating And Cooling Curve Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. For heating curves, we start with a solid and add heat energy. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Evaporation occurs when a liquid turns to a gas. Heating curves. Solid Heating And Cooling Curve.
From lessonschoolimbrowning.z14.web.core.windows.net
Heating Cooling Curve Calculator Solid Heating And Cooling Curve Heating curves relate temperature changes to phase transitions. An experiment can be planned to measure the temperature change through continuous heating of a substance. For heating curves, we start with a solid and add heat energy. Evaporation occurs when a liquid turns to a gas. Freezing is the opposite of melting, and both represent the equilibrium between the solid and. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Solid Heating And Cooling Curve Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. An experiment can be planned to measure the temperature change through continuous heating of a substance. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heating curves relate temperature changes to phase. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Solid Heating And Cooling Curve For cooling curves, we start with the gas phase and. Evaporation occurs when a liquid turns to a gas. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. An experiment can be planned to measure the temperature change through continuous heating of a substance. For heating curves,. Solid Heating And Cooling Curve.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Solid Heating And Cooling Curve The curve is divided into distinct segments, each corresponding to a specific phase of the substance. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. For cooling curves, we start with the gas phase and. Heating curves relate temperature changes to phase transitions. Freezing is the opposite. Solid Heating And Cooling Curve.
From byjus.com
Explain the cooling curve in crystalline and amorphous solids. Solid Heating And Cooling Curve An experiment can be planned to measure the temperature change through continuous heating of a substance. For cooling curves, we start with the gas phase and. For heating curves, we start with a solid and add heat energy. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. Heating curves relate temperature changes. Solid Heating And Cooling Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Solid Heating And Cooling Curve All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. Evaporation occurs when a liquid turns to a gas. Heat, cool and compress atoms and molecules and watch as they change. Solid Heating And Cooling Curve.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Solid Heating And Cooling Curve Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heating. Solid Heating And Cooling Curve.
From materialdbhutchins.z21.web.core.windows.net
What Are Heating And Cooling Curves Solid Heating And Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. For cooling curves, we start with the gas phase and. All of the changes of state that occur between solid, liquid, and. Solid Heating And Cooling Curve.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. An experiment can be planned to measure the temperature change through continuous heating of a substance. For heating curves, we start with a solid and add heat energy. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. All of the changes of state that. Solid Heating And Cooling Curve.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Solid Heating And Cooling Curve Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states. For heating curves, we start with a solid and add heat energy. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. A superheated liquid, a liquid at a temperature and. Solid Heating And Cooling Curve.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Solid Heating And Cooling Curve The curve is divided into distinct segments, each corresponding to a specific phase of the substance. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. For cooling curves, we start with the gas phase and. Heat, cool and compress atoms and molecules and watch as they change between solid,. Solid Heating And Cooling Curve.
From www.youtube.com
Cooling Curves for a LiquidSolid System YouTube Solid Heating And Cooling Curve The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Evaporation occurs when a liquid turns to a gas. Freezing is the opposite of melting, and both represent the equilibrium between the solid and liquid states.. Solid Heating And Cooling Curve.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Solid Heating And Cooling Curve Evaporation occurs when a liquid turns to a gas. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heating curves relate temperature changes to phase transitions. Heat, cool and compress atoms and molecules. Solid Heating And Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Solid Heating And Cooling Curve The curve is divided into distinct segments, each corresponding to a specific phase of the substance. Evaporation occurs when a liquid turns to a gas. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. For cooling curves, we start with the gas phase and. Freezing is the. Solid Heating And Cooling Curve.
From mungfali.com
Cooling Curve Labeled Solid Heating And Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Evaporation occurs when a liquid turns to a gas. The curve is divided into distinct segments, each corresponding to a specific phase of the substance. For heating curves, we start with a solid and add heat energy. Heating curves relate. Solid Heating And Cooling Curve.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Solid Heating And Cooling Curve An experiment can be planned to measure the temperature change through continuous heating of a substance. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. For heating curves, we start with a solid and add heat energy. A superheated liquid, a liquid at a temperature and pressure at which it should. Solid Heating And Cooling Curve.