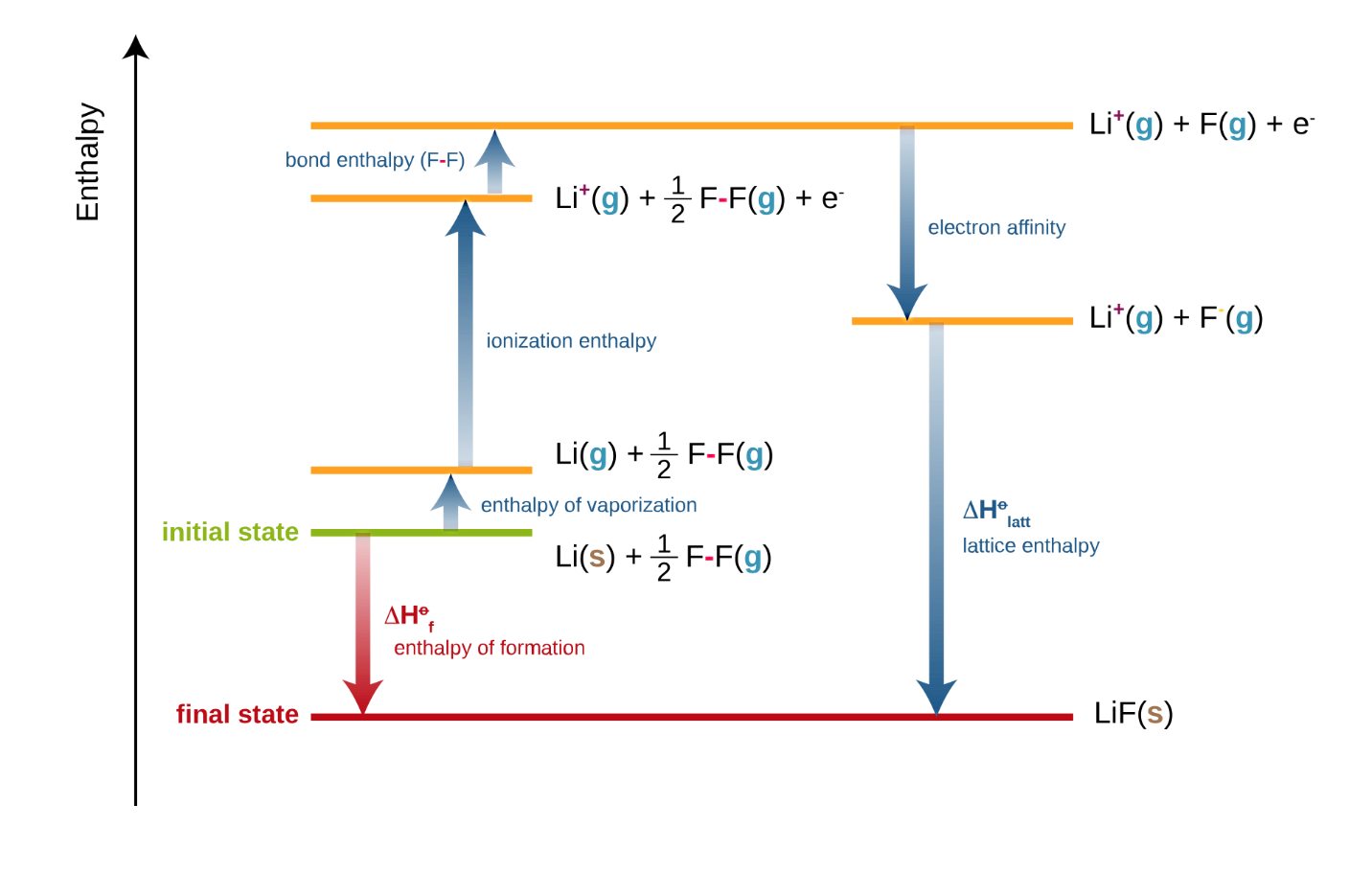
Standard Enthalpy Of Formation Sign . The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from www.w3schools.blog
For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is.
Standard enthalpy of ionization W3schools
Standard Enthalpy Of Formation Sign The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID305168 Standard Enthalpy Of Formation Sign The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. For example, although oxygen can exist as ozone (o 3),. Standard Enthalpy Of Formation Sign.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Similarly, hydrogen is h 2 (g),. Standard Enthalpy Of Formation Sign.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen. Standard Enthalpy Of Formation Sign.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Sign Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements. Standard Enthalpy Of Formation Sign.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Similarly, hydrogen is h 2 (g), not atomic. Standard Enthalpy Of Formation Sign.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Enthalpy Of Formation Sign Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. Standard Enthalpy Of Formation Sign.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is the enthalpy. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. 193 rows in chemistry and thermodynamics, the. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh _f$ is. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Sign Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Similarly, hydrogen. Standard Enthalpy Of Formation Sign.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard Enthalpy Of Formation Sign.
From www.studypool.com
SOLUTION Standard enthalpy of formation Studypool Standard Enthalpy Of Formation Sign The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh _f$ is an enthalpy. Standard Enthalpy Of Formation Sign.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Similarly, hydrogen is h 2. Standard Enthalpy Of Formation Sign.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and Standard Enthalpy Of Formation Sign The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation of any element in its standard state is zero by. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at. Standard Enthalpy Of Formation Sign.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is. Standard Enthalpy Of Formation Sign.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Similarly, hydrogen is h 2. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at.. Standard Enthalpy Of Formation Sign.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Sign The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Sign.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation of any element in its standard state is zero by. Standard Enthalpy Of Formation Sign.
From www.slideshare.net
2012 15 1 and 15 2 Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. For example, although oxygen can exist as. Standard Enthalpy Of Formation Sign.
From study.com
How to Draw & Label Enthalpy Diagrams Lesson Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at.. Standard Enthalpy Of Formation Sign.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Sign The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when. Standard Enthalpy Of Formation Sign.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Sign The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as. Standard Enthalpy Of Formation Sign.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Sign Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a. Standard Enthalpy Of Formation Sign.
From www.studypool.com
SOLUTION 5 CALCULATION OF ENTHALPY CHANGES OF REACTIONS USING Standard Enthalpy Of Formation Sign A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation of any. Standard Enthalpy Of Formation Sign.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. Similarly, hydrogen is h 2 (g), not atomic. Standard Enthalpy Of Formation Sign.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Sign Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in. Standard Enthalpy Of Formation Sign.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation Sign The standard enthalpy of formation of any element in its standard state is zero by definition. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Standard enthalpy of. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT STATE FUNCTIONS and ENTHALPY PowerPoint Presentation, free Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of any element in its standard state is zero by definition. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). 193 rows in. Standard Enthalpy Of Formation Sign.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Similarly, hydrogen is h 2 (g),. Standard Enthalpy Of Formation Sign.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at.. Standard Enthalpy Of Formation Sign.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Sign 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation of. Standard Enthalpy Of Formation Sign.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Sign For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm pressure and 25°c. Similarly, hydrogen is h 2 (g), not atomic hydrogen (h). The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in. Standard Enthalpy Of Formation Sign.