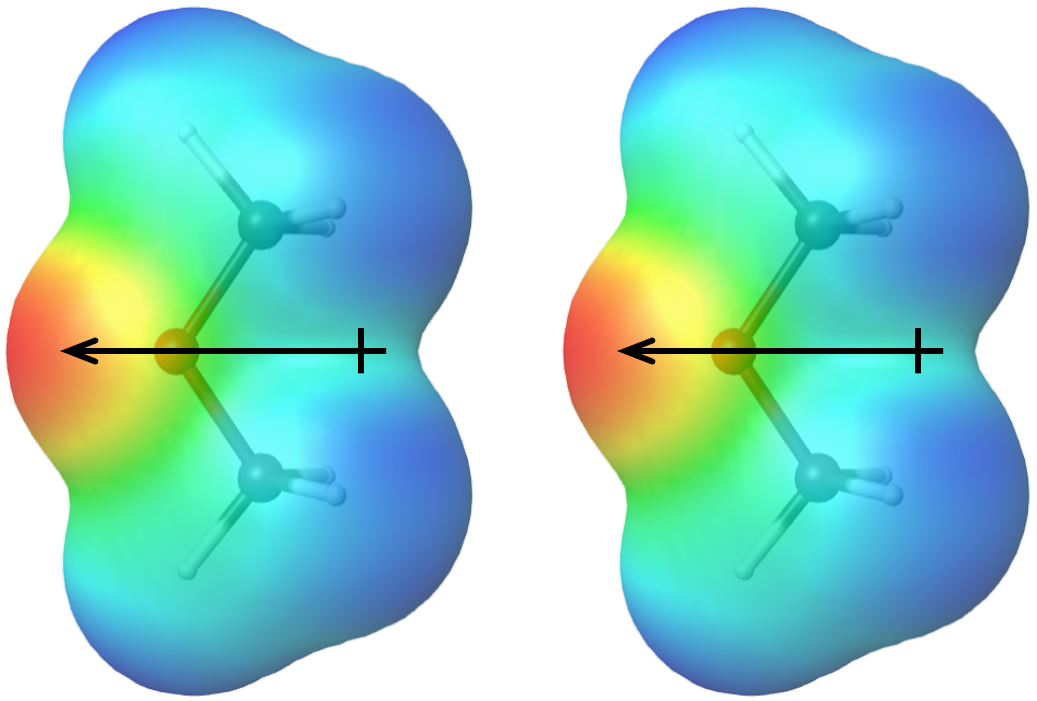
What Is A Dipole-Dipole Attraction . Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion.
from wisc.pb.unizin.org
Any molecule with a permanent. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a.
D13.2 Molecular Dipoles; DipoleDipole Attractions Chemistry 109 Fall
What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent.
From mavink.com
Examples Of Dipole Dipole Bonds What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 What Is A Dipole-Dipole Attraction Any molecule with a permanent. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.chemistrysteps.com
Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.youtube.com
7. Part 2 Temporary dipoledipole attractions YouTube What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From courses.lumenlearning.com
Dipole Moments MCC Organic Chemistry What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. When this occurs, the partially negative portion. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.breakingatom.com
Dipole Attraction What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.chemistrylearner.com
Dipoledipole Forces Definition and Examples What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.youtube.com
Dipole Dipole Forces of Attraction Intermolecular Forces YouTube What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.expii.com
Dipole Moment — Definition & Overview Expii What Is A Dipole-Dipole Attraction Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From wisc.pb.unizin.org
D13.2 Molecular Dipoles; DipoleDipole Attractions Chemistry 109 Fall What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.thoughtco.com
Learn What a Dipole Is in Chemistry and Physics What Is A Dipole-Dipole Attraction Any molecule with a permanent. The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 10 Structures of Solids and Liquids PowerPoint What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.expii.com
IonDipole Forces — Definition & Overview Expii What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From study.com
Hydrogen Bonding, DipoleDipole & IonDipole Forces Strong What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.chemistrystudent.com
Permanent DipoleDipole Forces (ALevel) ChemistryStudent What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From www.chemistrylearner.com
Iondipole Forces (Interaction) Definition and Examples What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From wirelibschweizer.z19.web.core.windows.net
How To Tell Dipole Dipole Forces What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From stock.adobe.com
Structure of Dipoleinduced dipole attractions Stock Vector Adobe Stock What Is A Dipole-Dipole Attraction Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From www.youtube.com
Dipoledipole Forces YouTube What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.wou.edu
CH103 Chapter 5 Covalent Bonds and Introduction to Organic Molecules What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.chemistrylearner.com
Dipoledipole Forces Definition and Examples What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From educationhealer.z21.web.core.windows.net
Permanent Dipole Induced Dipole What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Six Types 1) Dipole Dipole, 2) HydrogenBonding, 3) London What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.sliderbase.com
Intermolecular Forces of Attraction Presentation Chemistry What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From brainly.in
Explain dipole dipole forces Brainly.in What Is A Dipole-Dipole Attraction Any molecule with a permanent. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Intermolecular Attractions & the Properties of Liquids & Solids What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.chemistrylearner.com
Iondipole Forces (Interaction) Definition and Examples What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.
From chemistrymadesimple.net
The 3 Types of Intermolecular Forces of Attraction Chemistry Made Simple What Is A Dipole-Dipole Attraction Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID6769141 What Is A Dipole-Dipole Attraction Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint What Is A Dipole-Dipole Attraction The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. When this occurs, the partially negative portion. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID What Is A Dipole-Dipole Attraction Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. Any molecule with a permanent. When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. What Is A Dipole-Dipole Attraction.
From studyfurnishers.z4.web.core.windows.net
How To Draw A Dipole What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. What Is A Dipole-Dipole Attraction.
From www.slideserve.com
PPT Intermolecular Forces of Attraction PowerPoint Presentation, free What Is A Dipole-Dipole Attraction When this occurs, the partially negative portion. Recall from the chapter on chemical bonding and molecular geometry that polar molecules have a. The positive region of one molecule is attracted to the negative region of another. Any molecule with a permanent. What Is A Dipole-Dipole Attraction.