Inert Gas Equilibrium . So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. If you add an inert gas and allow the gas volume to change, then adding this gas. This will increase the total pressure of the. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with.
from www.slideshare.net
So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This will increase the total pressure of the. There are two cases on. If you add an inert gas and allow the gas volume to change, then adding this gas. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant.
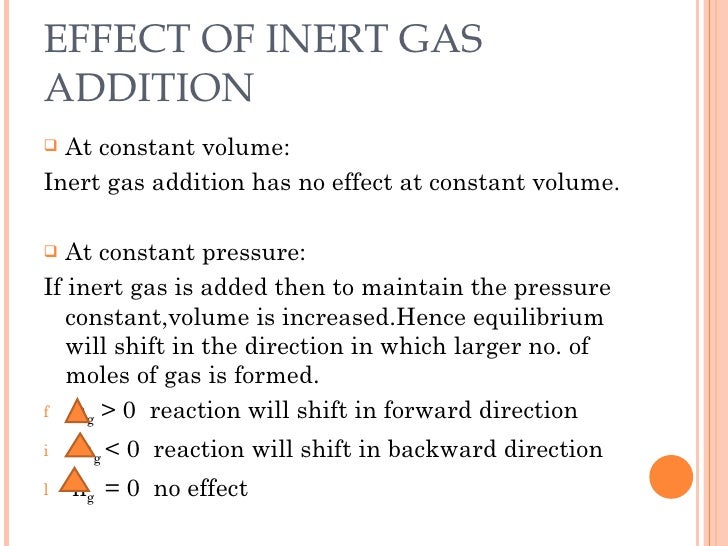
Chemical equillibrium
Inert Gas Equilibrium The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This video talks about what happens to the equilibrium when an inert gas is added at constant. This will increase the total pressure of the. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. If you add an inert gas and allow the gas volume to change, then adding this gas. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change.
From inertgaszaimono.blogspot.com
Inert Gas Effect Of Inert Gas On Equilibrium Inert Gas Equilibrium This will increase the total pressure of the. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. If you add an inert gas and allow the gas volume to change, then adding this gas. So, le chatelier’s principle applies when the partial pressure of a reactant. Inert Gas Equilibrium.
From www.numerade.com
SOLVEDAssertion Adding an inert gas todissociation equilibrium of Inert Gas Equilibrium So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the. There are two cases on. If you add an inert gas and allow the gas volume to change,. Inert Gas Equilibrium.
From www.youtube.com
The position of equilibrium will shift, by the addition of inert gas at Inert Gas Equilibrium This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an inert gas and allow the gas volume to change, then adding this gas. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the.. Inert Gas Equilibrium.
From www.youtube.com
Chemical Equilibrium Catalyst & Inert Gas Addition YouTube Inert Gas Equilibrium So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an. Inert Gas Equilibrium.
From inertgaszaimono.blogspot.com
Inert Gas Does Adding An Inert Gas Shift Equilibrium Inert Gas Equilibrium So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. If you add an inert gas and allow the gas volume to change, then adding this gas. This will increase the total pressure of the. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change.. Inert Gas Equilibrium.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Inert Gas Equilibrium Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. If you add an inert gas and allow the gas volume to change, then adding this gas. The addition of an. Inert Gas Equilibrium.
From www.chegg.com
Solved 1. Gas from a petroleum distillation column, has a Inert Gas Equilibrium There are two cases on. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This video talks about what happens to the equilibrium when an inert gas is added at constant. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. This. Inert Gas Equilibrium.
From www.numerade.com
SOLVED Question 3 (4 points) To increase the value of Kc in the Inert Gas Equilibrium There are two cases on. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This will increase the total pressure of the. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure,. Inert Gas Equilibrium.
From sciencenotes.org
Le Chatelier's Principle Inert Gas Equilibrium So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This video talks about what happens to the equilibrium when an inert gas is added at constant. There are two cases on. The addition of an. Inert Gas Equilibrium.
From www.bartleby.com
Answered If inert gas is added into the… bartleby Inert Gas Equilibrium This will increase the total pressure of the. There are two cases on. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. This video talks about what happens to the equilibrium when. Inert Gas Equilibrium.
From www.slideserve.com
PPT Ch. 13 Chemical Equilibrium PowerPoint Presentation, free Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. If you add an inert gas and allow the gas volume to change, then adding this gas. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or. Inert Gas Equilibrium.
From www.youtube.com
Inert Effect on Chemical Equilibrium YouTube Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. If you add an. Inert Gas Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Inert Gas Equilibrium This video talks about what happens to the equilibrium when an inert gas is added at constant. There are two cases on. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the. If you add an inert gas and allow the gas volume to. Inert Gas Equilibrium.
From www.youtube.com
EQUILIBRIUMEFFECT OF ADDITION OF INERT GAS YouTube Inert Gas Equilibrium So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. There are two cases on. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. This will increase the total pressure of the. Learn how a dynamic equilibrium shifts to counteract changes in. Inert Gas Equilibrium.
From www.youtube.com
Good Question Adding an inert gas to an equilibrium reaction YouTube Inert Gas Equilibrium Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. If you add an inert gas and allow the gas volume to change, then adding this gas. This will increase the. Inert Gas Equilibrium.
From www.toppr.com
Which among the following reac tion is true the following reac tion Inert Gas Equilibrium This will increase the total pressure of the. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. There are two cases on. This video talks about what happens to the equilibrium when an inert gas is added at constant. At otherwise the same partial pressure of the reacting gas, an. Inert Gas Equilibrium.
From byjus.com
36.At a certain temperature and 2 ATM pressure equilibrium constant KP Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. This will increase the total pressure of the. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. If you add an inert gas and allow the gas volume to change,. Inert Gas Equilibrium.
From www.youtube.com
effect of inert gas on equilibrium position (1) YouTube Inert Gas Equilibrium This will increase the total pressure of the. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an inert gas and allow the gas volume to change, then adding this. Inert Gas Equilibrium.
From www.doubtnut.com
Given the following reaction at equilibrium N(2)(g) + 3H(2)(g)hArr2NH(3 Inert Gas Equilibrium The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant. This will increase the total pressure of the. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous. Inert Gas Equilibrium.
From byjus.com
why on adding inert gas at constant volume there is no effect on state Inert Gas Equilibrium Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the. This video talks about what happens to the equilibrium when an inert gas is added at constant. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. At. Inert Gas Equilibrium.
From www.shutterstock.com
886 imágenes de Inert gases Imágenes, fotos y vectores de stock Inert Gas Equilibrium The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an inert gas and allow the gas volume to change, then adding this gas. This will increase the total pressure of. Inert Gas Equilibrium.
From www.youtube.com
On adding inert gas to equilibrium for given reaction at constant P Inert Gas Equilibrium Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. There are two cases on. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of. Inert Gas Equilibrium.
From www.toppr.com
of gas increases in the forward direction. 7.8.3 Effect of Inert Gas Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add. Inert Gas Equilibrium.
From inertgaszaimono.blogspot.com
Inert Gas Effect Of Inert Gas On Equilibrium At Constant Pressure Inert Gas Equilibrium There are two cases on. This video talks about what happens to the equilibrium when an inert gas is added at constant. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the. At otherwise the same partial pressure of the reacting gas, an inert. Inert Gas Equilibrium.
From byjus.com
why on adding inert gas at constant volume there is no effect on state Inert Gas Equilibrium This will increase the total pressure of the. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. There are two cases on. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. At otherwise the same partial pressure of the reacting gas, an inert. Inert Gas Equilibrium.
From www.youtube.com
Chemistry 11Chapter 7 Effect of Inert Gas Addition, Temperature Change Inert Gas Equilibrium The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. There are two cases on. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of. Inert Gas Equilibrium.
From edurev.in
What happens when an inert gas is added to an equilibrium keeping Inert Gas Equilibrium There are two cases on. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the total pressure of the. This video talks about what happens to the equilibrium when an inert gas. Inert Gas Equilibrium.
From byjus.com
35 What is d effect of addition of inert gas on equilibrium? Inert Gas Equilibrium This video talks about what happens to the equilibrium when an inert gas is added at constant. This will increase the total pressure of the. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions. Inert Gas Equilibrium.
From inertgaszaimono.blogspot.com
Inert Gas Effect Of Inert Gas On Equilibrium Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This will increase the total. Inert Gas Equilibrium.
From www.slideserve.com
PPT Equilibrium Concept Review PowerPoint Presentation, free download Inert Gas Equilibrium If you add an inert gas and allow the gas volume to change, then adding this gas. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. This video talks about what happens to the equilibrium when an inert gas is added at constant. This will increase the total. Inert Gas Equilibrium.
From www.youtube.com
LeChatelier's Principle Adding inert gas Equilibrium Chemistry Inert Gas Equilibrium This will increase the total pressure of the. There are two cases on. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an inert gas and allow the gas volume to change, then adding this gas. The addition of an inert gas can affect the equilbrium, but only if. Inert Gas Equilibrium.
From www.slideshare.net
Chemical equillibrium Inert Gas Equilibrium At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. The addition of an inert gas can affect the equilbrium, but only if the volume is allowed to change. There are two cases. Inert Gas Equilibrium.
From inertgaszaimono.blogspot.com
Inert Gas Inert Gas Le Chatelier''s Principle Inert Gas Equilibrium There are two cases on. This will increase the total pressure of the. So, le chatelier’s principle applies when the partial pressure of a reactant or product gas changes. This video talks about what happens to the equilibrium when an inert gas is added at constant. If you add an inert gas and allow the gas volume to change, then. Inert Gas Equilibrium.
From www.slideserve.com
PPT Equilibrium Concept PowerPoint Presentation, free download ID Inert Gas Equilibrium This video talks about what happens to the equilibrium when an inert gas is added at constant. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. If you add an inert gas and allow the gas volume to change, then adding this gas. There are two cases on. This will increase. Inert Gas Equilibrium.
From askfilo.com
Effect of Addition of Inert Gas Effect of Inert Gas on a Reaction at Equi.. Inert Gas Equilibrium If you add an inert gas and allow the gas volume to change, then adding this gas. At otherwise the same partial pressure of the reacting gas, an inert gas slows down heterogenous reactions of a gas with. Learn how a dynamic equilibrium shifts to counteract changes in temperature, pressure, or concentration of reactants or products. This will increase the. Inert Gas Equilibrium.