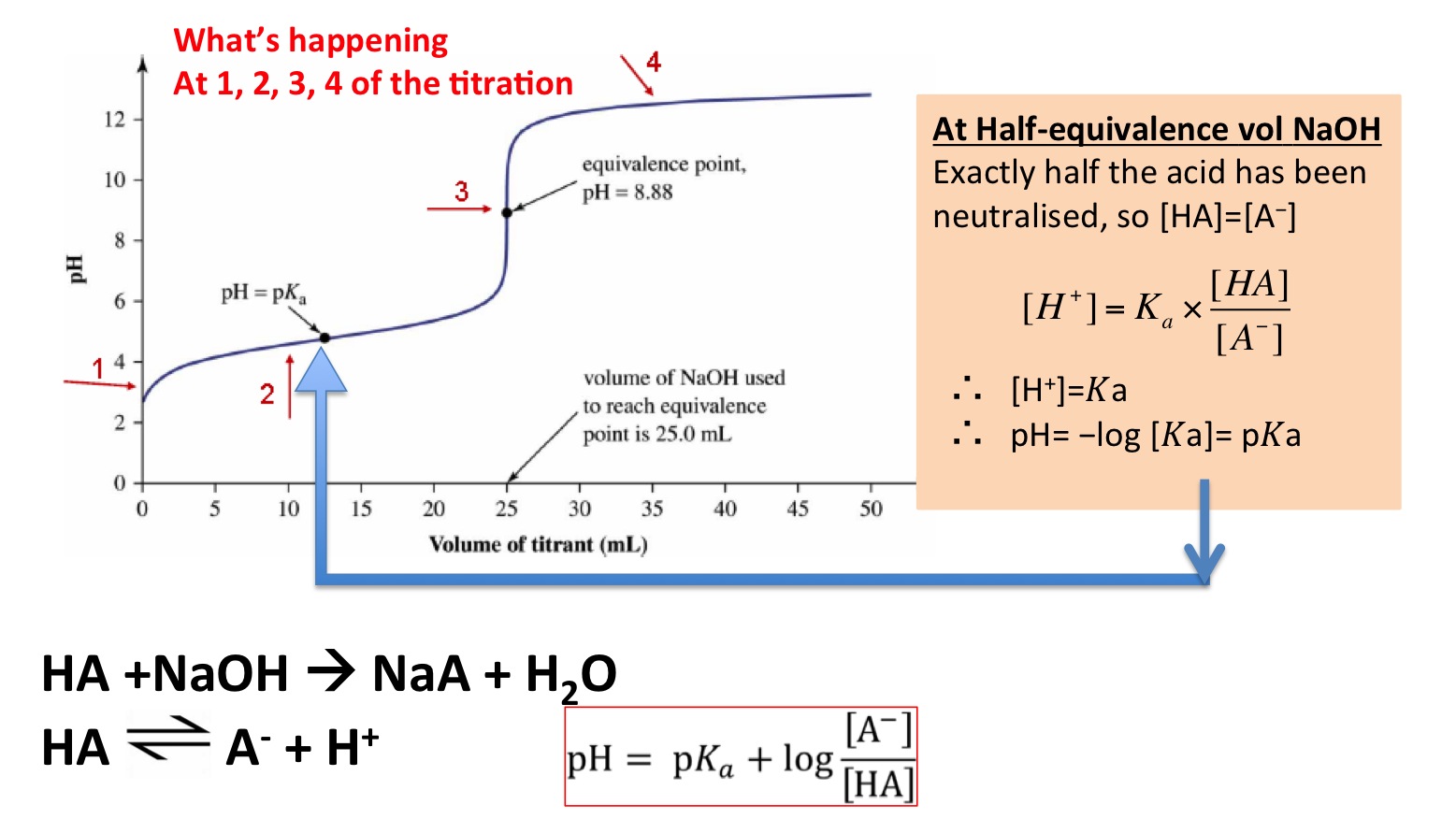
Titration Halfway Point . One point in the titration of a weak acid or a weak base is particularly important: This is also known as the equivalence point and this is. See titration curves for acids and bases. Titrating strong acids and strong bases. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. Compute sample ph at important stages of a titration. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Equivalence point → moles of alkali = moles of acid. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The equivalence point is halfway the vertical region of the curve. The equivalence point of a titration.
from socratic.org
The equivalence point of a titration. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. Compute sample ph at important stages of a titration. See titration curves for acids and bases. The equivalence point is halfway the vertical region of the curve. Titrating strong acids and strong bases. One point in the titration of a weak acid or a weak base is particularly important: Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Equivalence point → moles of alkali = moles of acid. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid.
The "pH" at onehalf the equivalence point in an acidbase titration
Titration Halfway Point Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Equivalence point → moles of alkali = moles of acid. Titrating strong acids and strong bases. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. One point in the titration of a weak acid or a weak base is particularly important: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Compute sample ph at important stages of a titration. The equivalence point is halfway the vertical region of the curve. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. This is also known as the equivalence point and this is. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. See titration curves for acids and bases. The equivalence point of a titration.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Halfway Point Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titrating strong acids and strong bases. Compute sample ph at important stages of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. See titration curves. Titration Halfway Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Halfway Point Compute sample ph at important stages of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. One point in the titration of a weak. Titration Halfway Point.
From www.youtube.com
7.9 the half equivalence point YouTube Titration Halfway Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. One point in the titration of a weak acid or a weak base is particularly important: Titrating strong acids and strong bases. The equivalence point of a titration. Equivalence point → moles of alkali =. Titration Halfway Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration Halfway Point This is also known as the equivalence point and this is. Titrating strong acids and strong bases. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. Equivalence point → moles of alkali = moles of acid. One point in the titration of a weak acid or a weak. Titration Halfway Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Halfway Point This is also known as the equivalence point and this is. Compute sample ph at important stages of a titration. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titrating strong acids and strong bases. The equivalence point is halfway the vertical region of the curve. The equivalence point of a titration.. Titration Halfway Point.
From www.chegg.com
Solved In this type of titration, halfway to the equivalence Titration Halfway Point This is also known as the equivalence point and this is. Compute sample ph at important stages of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added). Titration Halfway Point.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Titration Halfway Point See titration curves for acids and bases. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. Compute sample ph at important stages of a titration. The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is. Titration Halfway Point.
From www.chegg.com
Solved 1. Answer the following questions using the titration Titration Halfway Point This is also known as the equivalence point and this is. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The equivalence point of a titration. Titrating strong acids and strong bases. One point in the titration of a weak acid or a weak base is particularly important:. Titration Halfway Point.
From www.chegg.com
Solved This is an example of the titration halfway to the Titration Halfway Point For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. See titration curves for acids and bases. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. One point in the titration of a. Titration Halfway Point.
From www.youtube.com
Halfway Point in Titration YouTube Titration Halfway Point See titration curves for acids and bases. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. Compute sample ph at important stages of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The. Titration Halfway Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Titration Halfway Point Equivalence point → moles of alkali = moles of acid. This is also known as the equivalence point and this is. One point in the titration of a weak acid or a weak base is particularly important: See titration curves for acids and bases. Titrating strong acids and strong bases. In weak monoprotic acids, the point halfway between the beginning. Titration Halfway Point.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Titration Halfway Point For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. See titration curves for acids and bases. In weak monoprotic acids, the point halfway. Titration Halfway Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Halfway Point Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. This is also known as the equivalence point and this is. The equivalence point. Titration Halfway Point.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Halfway Point One point in the titration of a weak acid or a weak base is particularly important: Titrating strong acids and strong bases. This is also known as the equivalence point and this is. See titration curves for acids and bases. Compute sample ph at important stages of a titration. In weak monoprotic acids, the point halfway between the beginning of. Titration Halfway Point.
From www.numerade.com
SOLVED Calculate the pH at the halfway point and at the equivalence Titration Halfway Point Compute sample ph at important stages of a titration. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The equivalence point of a titration. The ph. Titration Halfway Point.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Titration Halfway Point For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Titration is an analytical chemistry technique used to find the concentration of an unknown. Titration Halfway Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Halfway Point See titration curves for acids and bases. Titrating strong acids and strong bases. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point of a titration. One point in the titration of a weak acid or a weak base is particularly important:. Titration Halfway Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Halfway Point The equivalence point of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. This is also known as the equivalence point and this is. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of. Titration Halfway Point.
From www.numerade.com
SOLVED You will observe a weak acidstrong base titration in this Titration Halfway Point Titrating strong acids and strong bases. Equivalence point → moles of alkali = moles of acid. This is also known as the equivalence point and this is. Compute sample ph at important stages of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The ph at. Titration Halfway Point.
From slideplayer.com
AcidBase Titrations Chapter ppt download Titration Halfway Point The equivalence point of a titration. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Compute sample ph at important stages of a titration. The equivalence point is halfway the vertical region of the curve. See titration curves for acids and bases. Equivalence point → moles of alkali = moles of acid.. Titration Halfway Point.
From www.chegg.com
Solved This is an example of the titration halfway to the Titration Halfway Point See titration curves for acids and bases. The equivalence point of a titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. One point in the. Titration Halfway Point.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Titration Halfway Point For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. The equivalence point is halfway the vertical region of the curve. Compute sample ph at important stages of a titration. The equivalence point of a titration. Titration is an analytical chemistry technique used to find the concentration of an. Titration Halfway Point.
From www.chegg.com
Solved Looking at your results in Graph 1 and comparing them Titration Halfway Point One point in the titration of a weak acid or a weak base is particularly important: In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. Titrating strong acids and strong bases. The ph at the midpoint, the point halfway on the titration curve to the equivalence point,. Titration Halfway Point.
From www.numerade.com
SOLVED Explain the difference between equivalence point and endpoint Titration Halfway Point This is also known as the equivalence point and this is. Equivalence point → moles of alkali = moles of acid. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. The equivalence point is halfway the vertical region of the curve. Compute sample ph at important stages. Titration Halfway Point.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Halfway Point The equivalence point is halfway the vertical region of the curve. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titrating strong acids and strong bases. Equivalence point → moles of alkali = moles of acid. This is also known as the equivalence point and this is. The ph at the midpoint,. Titration Halfway Point.
From www.chegg.com
Solved Calculate the pH at the halfway point and at the Titration Halfway Point This is also known as the equivalence point and this is. Titrating strong acids and strong bases. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. One point in the titration of a weak acid or a weak base is particularly important: For our first titration curve,. Titration Halfway Point.
From quizlet.com
Calculate the pH at the halfway point and at the equivalence Quizlet Titration Halfway Point In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. For our first titration curve, let’s consider the titration of 50.0 ml of. Titration Halfway Point.
From www.chegg.com
Solved Consider the titration curve for a diprotic acid 12 Titration Halfway Point Equivalence point → moles of alkali = moles of acid. This is also known as the equivalence point and this is. Compute sample ph at important stages of a titration. One point in the titration of a weak acid or a weak base is particularly important: See titration curves for acids and bases. Titration is an analytical chemistry technique used. Titration Halfway Point.
From slideplayer.com
AcidBase Titrations Chapter ppt download Titration Halfway Point One point in the titration of a weak acid or a weak base is particularly important: Titrating strong acids and strong bases. This is also known as the equivalence point and this is. The equivalence point of a titration. The equivalence point is halfway the vertical region of the curve. See titration curves for acids and bases. In weak monoprotic. Titration Halfway Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Halfway Point The equivalence point of a titration. Compute sample ph at important stages of a titration. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. This is also known as the. Titration Halfway Point.
From www.chegg.com
Solved Calculate the pH at the halfway point and at the Titration Halfway Point This is also known as the equivalence point and this is. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. Titrating strong acids and strong bases. One point in the titration of a weak acid or a weak base is particularly important: Equivalence point →. Titration Halfway Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Halfway Point Compute sample ph at important stages of a titration. Equivalence point → moles of alkali = moles of acid. See titration curves for acids and bases. Titrating strong acids and strong bases. One point in the titration of a weak acid or a weak base is particularly important: This is also known as the equivalence point and this is. For. Titration Halfway Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Halfway Point See titration curves for acids and bases. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200. In weak monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence. The equivalence point of a titration. Equivalence point → moles. Titration Halfway Point.
From www.numerade.com
SOLVED In the titration of 140.0 mL of 0.40 M HONHâ‚‚ with 0.20 M HBr Titration Halfway Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Compute sample ph at important stages of a titration. In weak monoprotic acids, the point halfway between the. Titration Halfway Point.
From www.numerade.com
SOLVEDConsider the following four titrations (1i v) a. Rank the Titration Halfway Point Equivalence point → moles of alkali = moles of acid. One point in the titration of a weak acid or a weak base is particularly important: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. In weak monoprotic acids, the point halfway between the. Titration Halfway Point.