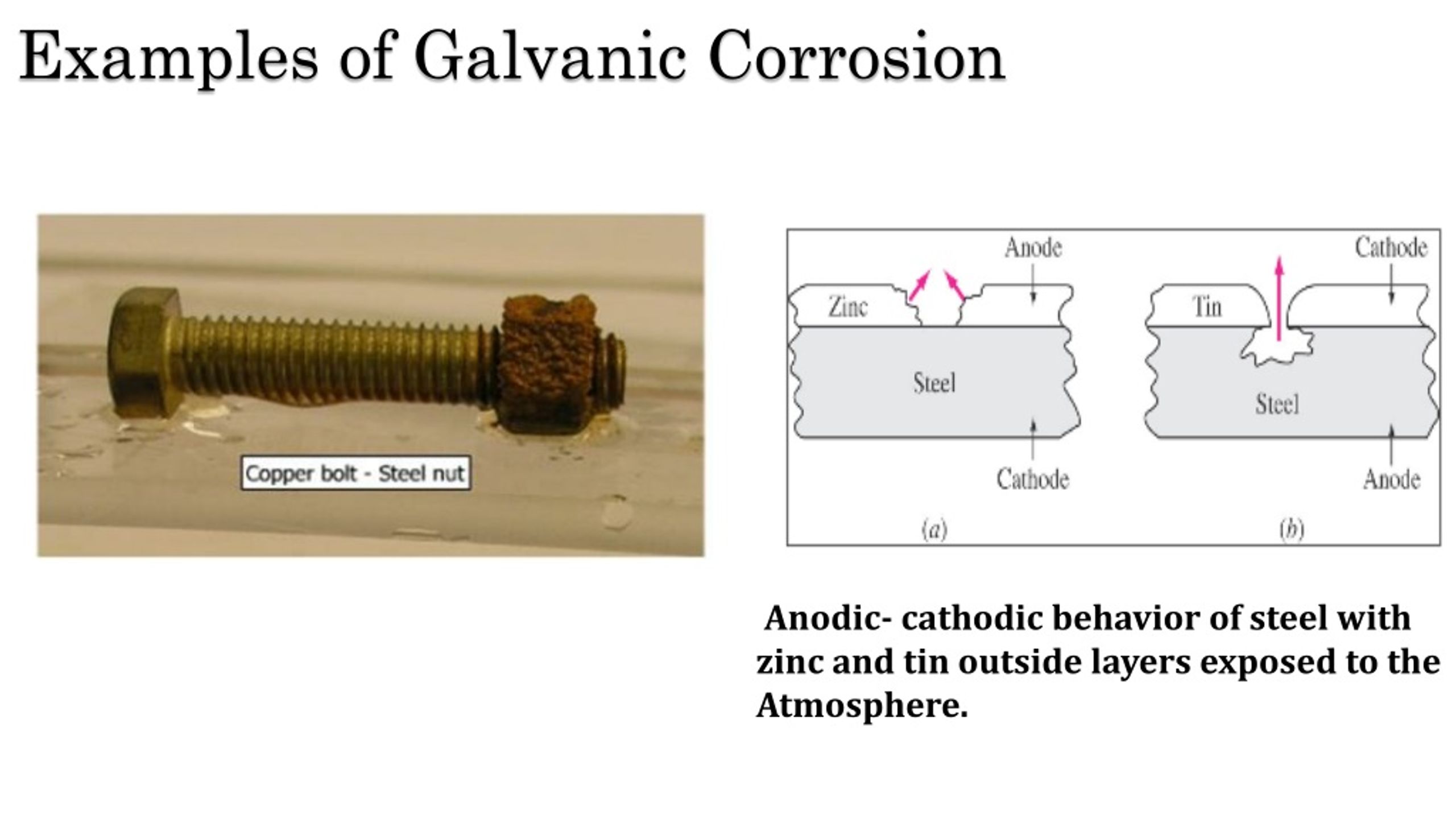
Examples Of Galvanic Corrosion . corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e.
from www.slideserve.com
galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides.
PPT CORROSION PowerPoint Presentation, free download ID290411
Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when.
From orapiasia.com
Galvanic Corrosion An InDepth Analysis ORAPI Asia Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when.. Examples Of Galvanic Corrosion.
From steelfabservices.com.au
Your Guide To Treating Galvanic Corrosion Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of. Examples Of Galvanic Corrosion.
From blog.thepipingmart.com
Galvanic Corrosion of Brass Steel Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. galvanic corrosion,. Examples Of Galvanic Corrosion.
From galvanic-isolator.co.uk
Galvanic Corrosion Examples Galvanic Isolators Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading. Examples Of Galvanic Corrosion.
From www.researchgate.net
Galvanic corrosion of the carbon steel wall adjacent to the stainless Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal. Examples Of Galvanic Corrosion.
From mh-mechanicalengineering.blogspot.com
Mechanical Engineering Galvanic Corrosion and Stainless Steel Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected,. Examples Of Galvanic Corrosion.
From steelfabservices.com.au
How To Protect From Steel Corrosion Steel Fab Services Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of. Examples Of Galvanic Corrosion.
From www.alideck.co.uk
Galvanic Corrosion » AliDeck Examples Of Galvanic Corrosion galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. numerous examples of galvanic corrosion are. Examples Of Galvanic Corrosion.
From www.corrosionclinic.com
Different Forms of Corrosion Galvanic Corrosion, Bimetallic Corrosion Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. corrosion is a galvanic process. Examples Of Galvanic Corrosion.
From www.ssina.com
Galvanic Corrosion SSINA Examples Of Galvanic Corrosion galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion,. Examples Of Galvanic Corrosion.
From mavink.com
Pipe Galvanic Corrosion Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of. Examples Of Galvanic Corrosion.
From www.slideserve.com
PPT CORROSION PowerPoint Presentation, free download ID290411 Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to. Examples Of Galvanic Corrosion.
From www.slideserve.com
PPT CORROSION PowerPoint Presentation, free download ID290411 Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected,. Examples Of Galvanic Corrosion.
From www.intermarineboats.com
Galvanic Corrosion Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their. Examples Of Galvanic Corrosion.
From mungfali.com
Galvanic Corrosion Examples Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading. Examples Of Galvanic Corrosion.
From www.icorr.org
Galvanic Corrosion the importance of designingout corrosion hotspot Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. numerous examples of galvanic corrosion are encountered. Examples Of Galvanic Corrosion.
From www.slideshare.net
Galvanic corrosion Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms. Examples Of Galvanic Corrosion.
From galvanizeit.org
Galvanic Corrosion & Galvanic Cell… American Galvanizers Association Examples Of Galvanic Corrosion galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion,. Examples Of Galvanic Corrosion.
From www.eboss.co.nz
Galvanic Action Mismatching of Corrosion Protected Steel Products EBOSS Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the. Examples Of Galvanic Corrosion.
From crossroadsgalvanizing.com
Galvanic Corrosion What is it? How to Prevent it? Crossroads Galvanizing Examples Of Galvanic Corrosion corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e.. Examples Of Galvanic Corrosion.
From chicagocorrosiongroup.com
Galvanic corrosion exposed on the streets of Dubrovnik Chicago Examples Of Galvanic Corrosion galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. numerous examples of galvanic corrosion are. Examples Of Galvanic Corrosion.
From civiconcepts.com
12 Types Of Corrosion With Pictures Examples Of Galvanic Corrosion galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. corrosion is. Examples Of Galvanic Corrosion.
From captaincorrosion.com
Galvanic Corrosion Simulator Captain Corrosion Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected,. Examples Of Galvanic Corrosion.
From galvanic-isolator.co.uk
Galvanic Corrosion Examples Galvanic Isolators Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. corrosion is a galvanic process. Examples Of Galvanic Corrosion.
From galvanizeit.org
Dissimilar Metal Corrosion with… American Galvanizers Association Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms. Examples Of Galvanic Corrosion.
From info.vantagepointroofing.com.au
The difference between rust and galvanic corrosion Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of. Examples Of Galvanic Corrosion.
From galvanic-isolator.co.uk
Galvanic Corrosion Examples Galvanic Isolators Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. corrosion is a galvanic process. Examples Of Galvanic Corrosion.
From engineerexcel.com
Galvanic Corrosion [with Chart] EngineerExcel Examples Of Galvanic Corrosion the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal. Examples Of Galvanic Corrosion.
From chicagocorrosiongroup.com
Galvanic corrosion exposed on the streets of Dubrovnik Chicago Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their. Examples Of Galvanic Corrosion.
From blog.thepipingmart.com
What is Galvanic Corrosion? Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to. Examples Of Galvanic Corrosion.
From blog.thepipingmart.com
Galvanic Corrosion vs Electrolysis What's the Difference Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the. Examples Of Galvanic Corrosion.
From www.slideserve.com
PPT CORROSION PowerPoint Presentation ID3003961 Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not always to their oxides. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. the increased. Examples Of Galvanic Corrosion.
From westernspecialtycontractors.com
Dissimilar Metals & Galvanic Corrosion Western Specialty Contractors Examples Of Galvanic Corrosion numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of. Examples Of Galvanic Corrosion.
From www.slideserve.com
PPT Galvanic corrosion PowerPoint Presentation, free download ID350961 Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. galvanic corrosion is also known as bimetallic corrosion, and during this process, the positively charged metal (the cathode) is unprotected, leading to corrosion, while the reacting negatively charged metal (the anode) is protected. the increased corrosion of the anode. Examples Of Galvanic Corrosion.
From steelfabservices.com.au
Your Guide To Treating Galvanic Corrosion Examples Of Galvanic Corrosion galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when. numerous examples of galvanic corrosion are encountered as it is one of the most frequently encountered forms of corrosion. the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of. Examples Of Galvanic Corrosion.