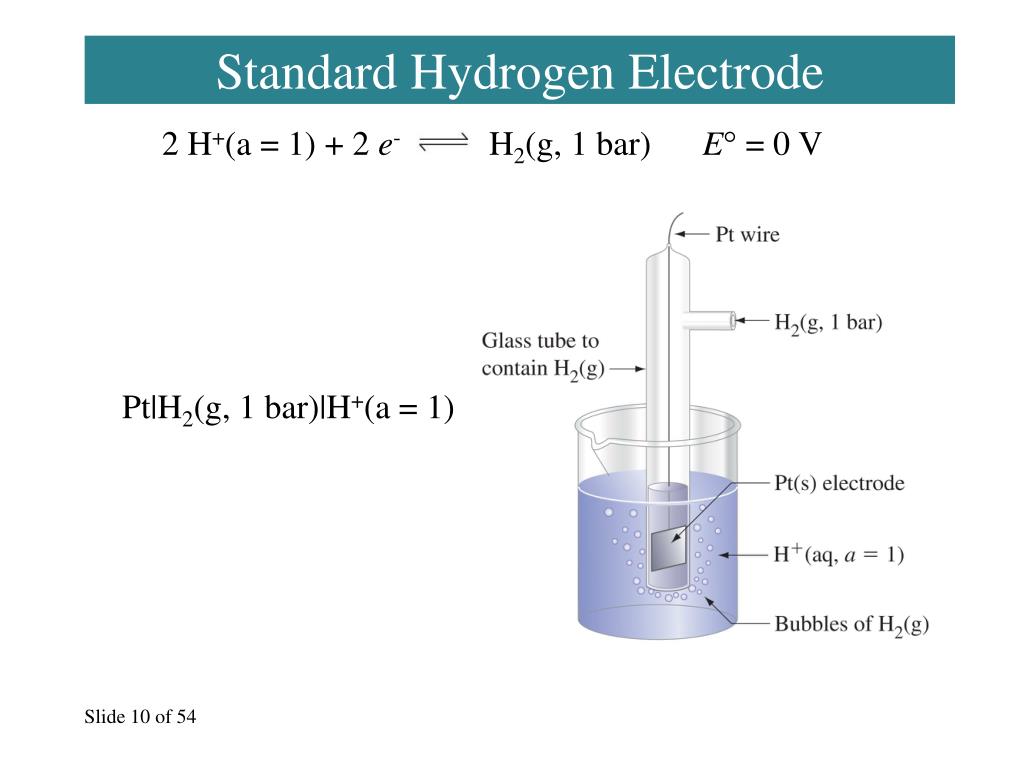
Hydrogen Electrode Standard Potential . The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.
from www.slideserve.com
The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard.
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free
Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. This is because it acts as a reference for comparison with any other electrode. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.
From pandai.me
Standard Electrode Potential Hydrogen Electrode Standard Potential The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox. Hydrogen Electrode Standard Potential.
From www.chemistryland.com
Lab 8 Single Replacement Reactions Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison. Hydrogen Electrode Standard Potential.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a Hydrogen Electrode Standard Potential In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Graphical representation of the onset potentials of. Hydrogen Electrode Standard Potential.
From gaskatel.de
Reference electrode Gaskatel Hydrogen Electrode Standard Potential The structure of the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. Graphical representation of the onset potentials of the her/hor and oer/orr measured. Hydrogen Electrode Standard Potential.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is. Hydrogen Electrode Standard Potential.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Hydrogen Electrode Standard Potential The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. The structure of the standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of. Hydrogen Electrode Standard Potential.
From 2012books.lardbucket.org
Standard Potentials Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic.. Hydrogen Electrode Standard Potential.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode Standard Potential The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode Standard Potential This is because it acts as a reference for comparison with any other electrode. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode Standard Potential This is because it acts as a reference for comparison with any other electrode. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen.. Hydrogen Electrode Standard Potential.
From mccord.cm.utexas.edu
Standard Potential Hydrogen Electrode Standard Potential In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode. Hydrogen Electrode Standard Potential.
From fyoxffenv.blob.core.windows.net
Standard Electrode Potential Positive Or Negative at David Means blog Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode Standard Potential In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared. Hydrogen Electrode Standard Potential.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode Standard Potential The structure of the standard. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms. Hydrogen Electrode Standard Potential.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Hydrogen Electrode Standard Potential The structure of the standard. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. 372 rows the data below tabulates standard electrode potentials (e°),. Hydrogen Electrode Standard Potential.
From www.researchgate.net
Absolute and relative (respect to Standard Hydrogen Electrode, SHE Hydrogen Electrode Standard Potential The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard. Hydrogen Electrode Standard Potential.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Standard Potential The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. This is because it acts as a reference for comparison with any other electrode. In electrochemistry,. Hydrogen Electrode Standard Potential.
From gaskatel.de
Reference electrode Gaskatel Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard. This is because it acts as a reference for comparison with any other electrode.. Hydrogen Electrode Standard Potential.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that Hydrogen Electrode Standard Potential This is because it acts as a reference for comparison with any other electrode. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard. The standard. Hydrogen Electrode Standard Potential.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose. Hydrogen Electrode Standard Potential.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Hydrogen Electrode Standard Potential The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox. Hydrogen Electrode Standard Potential.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. This is because it acts as a reference for comparison with any other electrode. The structure of the standard. The standard. Hydrogen Electrode Standard Potential.
From saylordotorg.github.io
Standard Potentials Hydrogen Electrode Standard Potential The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The. Hydrogen Electrode Standard Potential.
From askfilo.com
3.4. Calculate the potential of hydrogen electrode in contact with a solu.. Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v.. Hydrogen Electrode Standard Potential.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The. Hydrogen Electrode Standard Potential.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Standard Potential This is because it acts as a reference for comparison with any other electrode. The structure of the standard. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen.. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Hydrogen Electrode Standard Potential.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode Hydrogen Electrode Standard Potential The structure of the standard. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen. Hydrogen Electrode Standard Potential.
From glossary.periodni.com
Redokspotencijal Kemijski rječnik & glosar Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms. Hydrogen Electrode Standard Potential.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard. Hydrogen Electrode Standard Potential.
From app.pandai.org
Standard Electrode Potential Hydrogen Electrode Standard Potential The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard. This is because it acts as a reference for comparison with. Hydrogen Electrode Standard Potential.
From psu.pb.unizin.org
Electrode and Cell Potentials (17.3) Chemistry 110 Hydrogen Electrode Standard Potential Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the. Hydrogen Electrode Standard Potential.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Hydrogen Electrode Standard Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen.. Hydrogen Electrode Standard Potential.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Hydrogen Electrode Standard Potential In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.. Hydrogen Electrode Standard Potential.