White Solid Turns Yellow When Heated . in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. reacts as a powder on strong heating. It's brown when it's hot and turns yellow when it's cool. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. lead oxide is a peculiar type of salt. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. Zinc (zn) reacts steadily when heated forming a yellow solid which. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. The residue on heating turns yellow.
from www.snexplores.org
The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. It's brown when it's hot and turns yellow when it's cool. reacts as a powder on strong heating. lead oxide is a peculiar type of salt. The residue on heating turns yellow. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s.
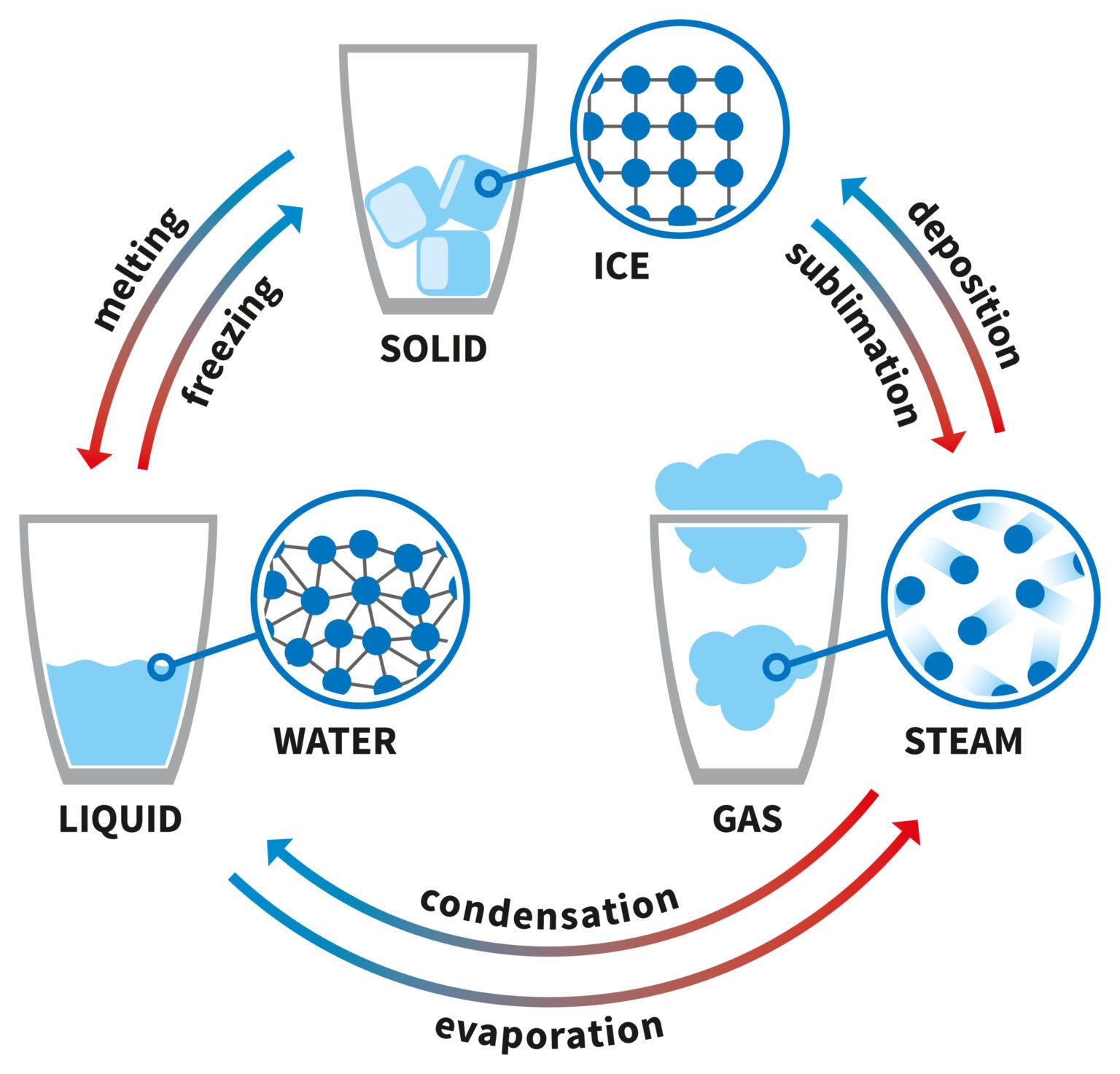
Explainer What are the different states of matter?
White Solid Turns Yellow When Heated It's brown when it's hot and turns yellow when it's cool. Zinc (zn) reacts steadily when heated forming a yellow solid which. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. lead oxide is a peculiar type of salt. It's brown when it's hot and turns yellow when it's cool. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The residue on heating turns yellow. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. reacts as a powder on strong heating. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk White Solid Turns Yellow When Heated It's brown when it's hot and turns yellow when it's cool. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The excess zn ions thus formed get entrapped in the. White Solid Turns Yellow When Heated.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric sulfate pentahydrate White Solid Turns Yellow When Heated lead oxide is a peculiar type of salt. It's brown when it's hot and turns yellow when it's cool. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The residue on heating turns yellow. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance. White Solid Turns Yellow When Heated.
From dxoeystgc.blob.core.windows.net
Gas In Liquid Or Solid at William Derr blog White Solid Turns Yellow When Heated reacts as a powder on strong heating. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. lead oxide is a peculiar type of salt. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the. White Solid Turns Yellow When Heated.
From scozzatobmschematic.z14.web.core.windows.net
What Happens To The Particles When Heated White Solid Turns Yellow When Heated reacts as a powder on strong heating. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The residue on heating turns yellow. lead oxide is a peculiar type of salt. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the. White Solid Turns Yellow When Heated.
From www.doubtnut.com
A salt (A) when heated with K2 Cr2 O7 and conc. H2 SO4 liberates a gas White Solid Turns Yellow When Heated The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. It's brown when it's hot and turns yellow when it's cool. Zinc (zn) reacts steadily when heated forming a yellow solid which. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. . White Solid Turns Yellow When Heated.
From www.toppr.com
The yellow colour or ZnO and conducting nature produced in heating is White Solid Turns Yellow When Heated Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. reacts as a powder on strong heating. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. The residue on heating turns yellow. lead oxide is a peculiar type of salt. It's. White Solid Turns Yellow When Heated.
From circuitlibrarybauer.z13.web.core.windows.net
Solid Liquid Gas Diagram White Solid Turns Yellow When Heated Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. reacts as a powder on strong heating. Zinc (zn) reacts steadily when heated forming a yellow solid which. It's brown when it's. White Solid Turns Yellow When Heated.
From mavink.com
Steel Temperature Color Chart White Solid Turns Yellow When Heated Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. It's brown when it's hot and turns yellow when it's cool. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the. White Solid Turns Yellow When Heated.
From allofkitchen.com
How To Remove Blue Loctite? White Solid Turns Yellow When Heated out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. reacts as a powder on strong heating. lead oxide is a peculiar type of salt. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. in $\ce{zno}$, $\ce{zn}$ is present in. White Solid Turns Yellow When Heated.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts White Solid Turns Yellow When Heated It's brown when it's hot and turns yellow when it's cool. lead oxide is a peculiar type of salt. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state,. White Solid Turns Yellow When Heated.
From gardensofthesun.com
Why does your white gold turn yellow? And how to fix it? (updated 2023 White Solid Turns Yellow When Heated Zinc (zn) reacts steadily when heated forming a yellow solid which. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. reacts as a powder on strong heating. The residue. White Solid Turns Yellow When Heated.
From www.snexplores.org
Explainer What are the different states of matter? White Solid Turns Yellow When Heated Zinc (zn) reacts steadily when heated forming a yellow solid which. reacts as a powder on strong heating. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. The residue on heating turns yellow. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped. White Solid Turns Yellow When Heated.
From dxoqlkmys.blob.core.windows.net
Solid Liquid Gas Temperature at Eliseo Roberts blog White Solid Turns Yellow When Heated reacts as a powder on strong heating. It's brown when it's hot and turns yellow when it's cool. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. Zinc. White Solid Turns Yellow When Heated.
From vendingproservice.com
Why does white corn turn yellow when boiled Vending Business Machine White Solid Turns Yellow When Heated reacts as a powder on strong heating. The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated forming a yellow solid which. It's brown when it's hot and turns yellow when it's cool. in $\ce{zno}$, $\ce{zn}$ is. White Solid Turns Yellow When Heated.
From byjus.com
Sublimation (Phase Transition) Definition & Examples with Videos White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The residue on heating turns yellow. It's brown when it's hot and turns yellow when it's cool. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The excess zn ions. White Solid Turns Yellow When Heated.
From home.techinfus.com
What to do if the water from the well turns yellow in air and when heated White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. Zinc (zn) reacts steadily when heated forming a yellow solid which. The residue on heating turns yellow. reacts as a powder on strong heating. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white. White Solid Turns Yellow When Heated.
From studylib.net
2 Unit 5 White Solid Turns Yellow When Heated The residue on heating turns yellow. lead oxide is a peculiar type of salt. reacts as a powder on strong heating. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and. White Solid Turns Yellow When Heated.
From dxogzljcw.blob.core.windows.net
Solid Changes To Liquid Is Called at Sharon Smith blog White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. out. White Solid Turns Yellow When Heated.
From www.youtube.com
`ZnO` is white cold and yellow when heated, it is due to the White Solid Turns Yellow When Heated Zinc (zn) reacts steadily when heated forming a yellow solid which. lead oxide is a peculiar type of salt. The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell. White Solid Turns Yellow When Heated.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. reacts as a powder on strong heating. The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated. White Solid Turns Yellow When Heated.
From socratic.org
The color of light absorbed by an aqueous solution of CuSO_"4" is White Solid Turns Yellow When Heated reacts as a powder on strong heating. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zno turns yellow on heating as zn2+ ions move in interstitial sites. White Solid Turns Yellow When Heated.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. Zinc (zn) reacts steadily when heated forming a yellow solid which. The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. reacts as. White Solid Turns Yellow When Heated.
From www.numerade.com
SOLVED In the table below, there are descriptions of an experiment on White Solid Turns Yellow When Heated The residue on heating turns yellow. reacts as a powder on strong heating. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. Zinc (zn) reacts steadily when heated forming a yellow solid which. Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped. White Solid Turns Yellow When Heated.
From www.doubtnut.com
metal deficiency defect due to absence of positive ions White Solid Turns Yellow When Heated The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. reacts as a powder on strong heating. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The residue on heating turns yellow. Zno turns yellow on heating as. White Solid Turns Yellow When Heated.
From www.alamy.com
Zinc oxide being heated in the flame of a bunsen burner. When heated White Solid Turns Yellow When Heated Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. The residue on heating turns yellow. It's brown when it's hot and turns yellow when it's cool. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated. White Solid Turns Yellow When Heated.
From askfilo.com
When a solid is heated, it turns directly into a gas. This process is cal.. White Solid Turns Yellow When Heated out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated forming a yellow solid which. lead oxide is a peculiar type of salt. It's brown when. White Solid Turns Yellow When Heated.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The White Solid Turns Yellow When Heated It's brown when it's hot and turns yellow when it's cool. Zinc (zn) reacts steadily when heated forming a yellow solid which. The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zno turns yellow on heating as zn2+ ions move in interstitial sites and. White Solid Turns Yellow When Heated.
From exobvyetp.blob.core.windows.net
What Is Gas To Solid Give An Example at Alice Denzer blog White Solid Turns Yellow When Heated Zno turns yellow on heating as zn2+ ions move in interstitial sites and electrons also get entrapped in. Zinc (zn) reacts steadily when heated forming a yellow solid which. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and. White Solid Turns Yellow When Heated.
From www.youtube.com
A pale yellow substance `(A)` when heated with `conc. HNO_3` evolves a White Solid Turns Yellow When Heated The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. reacts as a powder on strong heating. Zinc (zn) reacts steadily when heated forming a yellow solid which. lead oxide is a peculiar type of salt. It's brown when it's hot and turns yellow when it's cool. in. White Solid Turns Yellow When Heated.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science White Solid Turns Yellow When Heated lead oxide is a peculiar type of salt. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. It's brown when it's hot and turns yellow when it's cool. reacts as a powder on strong heating.. White Solid Turns Yellow When Heated.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. It's brown when it's hot and turns yellow when it's cool. lead oxide is a peculiar type of salt.. White Solid Turns Yellow When Heated.
From www.youtube.com
To show that solids, liquids, and gases expand on heating YouTube White Solid Turns Yellow When Heated The residue on heating turns yellow. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white. White Solid Turns Yellow When Heated.
From www.toppr.com
ZnO is white when cold and yellow when heated. It is due to development of White Solid Turns Yellow When Heated The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated forming a yellow solid which. in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. It's brown when it's hot and turns yellow when. White Solid Turns Yellow When Heated.
From www.wisegeek.com
What is Thermodynamics? (with pictures) White Solid Turns Yellow When Heated out of all the options, lead carbonate \[\left( pbc{{o}_{3}} \right)\] is a white solid substance which when. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. Zinc (zn) reacts steadily when heated forming a yellow solid which. lead oxide is a peculiar type of salt. Zno turns yellow. White Solid Turns Yellow When Heated.
From www.pmpa.org
Heat Treat Colors Of Steel Chart White Solid Turns Yellow When Heated in $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s. The excess zn ions thus formed get entrapped in the interstitial site and electron in the neighborhood vacant interstitial. lead oxide is a peculiar type of salt. It's brown when it's hot and turns yellow when it's cool.. White Solid Turns Yellow When Heated.