Does Hcl Exhibit Hydrogen Bonding . Even though chlorine is highly electronegative, the best answer is no, and in. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. does chlorine form hydrogen bonds? despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; The donor atom (dn), the hydrogen itself (h) and an. a hydrogen bond by definition needs three centers to be formed: Such a bond is weaker than an ionic bond. Any molecule which has a hydrogen atom attached directly to an.
from www.numerade.com
does chlorine form hydrogen bonds? The donor atom (dn), the hydrogen itself (h) and an. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. Even though chlorine is highly electronegative, the best answer is no, and in. a hydrogen bond by definition needs three centers to be formed: Any molecule which has a hydrogen atom attached directly to an. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Such a bond is weaker than an ionic bond. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient.
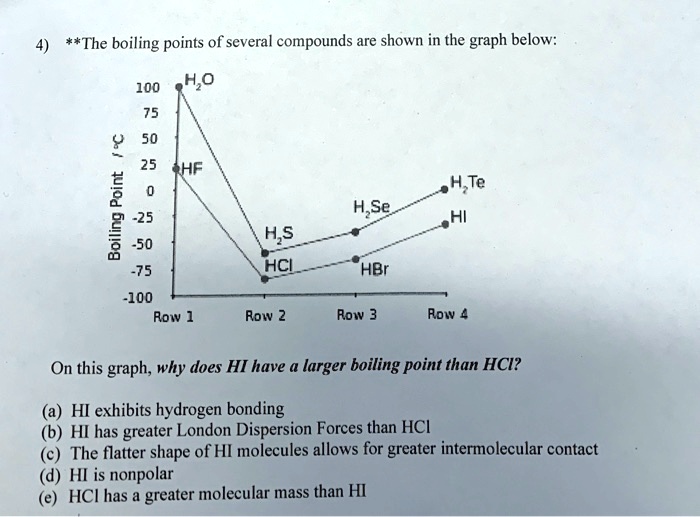
SOLVED The boiling points of several compounds are shown in the graph below 100 H2O 50 25 HF 2
Does Hcl Exhibit Hydrogen Bonding Any molecule which has a hydrogen atom attached directly to an. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. does chlorine form hydrogen bonds? any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Even though chlorine is highly electronegative, the best answer is no, and in. Any molecule which has a hydrogen atom attached directly to an. a hydrogen bond by definition needs three centers to be formed: Such a bond is weaker than an ionic bond. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. The donor atom (dn), the hydrogen itself (h) and an. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons;
From www.chegg.com
Solved Which molecule(s) exhibit hydrogen bonding? CH4 Does Hcl Exhibit Hydrogen Bonding Such a bond is weaker than an ionic bond. Even though chlorine is highly electronegative, the best answer is no, and in. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. does chlorine form hydrogen bonds? any molecule which has a hydrogen atom attached directly to an oxygen or a. Does Hcl Exhibit Hydrogen Bonding.
From www.youtube.com
HCl Molecular Geometry / Shape and Bond Angles (hydrochloric acid) YouTube Does Hcl Exhibit Hydrogen Bonding Even though chlorine is highly electronegative, the best answer is no, and in. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; a hydrogen bond by definition needs three centers to be formed: Such a bond is weaker than an ionic bond. Any molecule which has a. Does Hcl Exhibit Hydrogen Bonding.
From www.science-revision.co.uk
Electronegativity and polar bonds Does Hcl Exhibit Hydrogen Bonding does chlorine form hydrogen bonds? despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. The donor atom (dn), the hydrogen itself (h) and an. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Even though chlorine is. Does Hcl Exhibit Hydrogen Bonding.
From www.alamy.com
Covalent bond of hydrogen chlorine (HCl). Physics resources for teachers and students Stock Does Hcl Exhibit Hydrogen Bonding any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. The. Does Hcl Exhibit Hydrogen Bonding.
From www.pw.live
Hydrogen Bond, Types Of Hydrogen Bonding, Important Factors PW Does Hcl Exhibit Hydrogen Bonding Any molecule which has a hydrogen atom attached directly to an. The donor atom (dn), the hydrogen itself (h) and an. a hydrogen bond by definition needs three centers to be formed: the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. Such a bond is weaker than an ionic bond. . Does Hcl Exhibit Hydrogen Bonding.
From www.slideserve.com
PPT Molecular Structure & Intermolecular Forces PowerPoint Presentation ID5094369 Does Hcl Exhibit Hydrogen Bonding Even though chlorine is highly electronegative, the best answer is no, and in. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Any molecule which has a hydrogen atom. Does Hcl Exhibit Hydrogen Bonding.
From www.youtube.com
Hydrogen Bonding YouTube Does Hcl Exhibit Hydrogen Bonding Even though chlorine is highly electronegative, the best answer is no, and in. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. a hydrogen bond by definition needs three centers to be formed: does chlorine form hydrogen bonds? hydrogen bonding, interaction involving a hydrogen atom located between. Does Hcl Exhibit Hydrogen Bonding.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills Does Hcl Exhibit Hydrogen Bonding does chlorine form hydrogen bonds? despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other. Does Hcl Exhibit Hydrogen Bonding.
From www.slideshare.net
Intermolecular Forces in Hydrogen Bonding Pooja N Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; The donor atom (dn), the hydrogen itself (h) and an. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Any molecule which has a hydrogen atom attached. Does Hcl Exhibit Hydrogen Bonding.
From mydigitalkemistry.com
Hydrogen Bonding Definition ,Examples and Types Digital Kemistry Best Online Free Chemistry Does Hcl Exhibit Hydrogen Bonding despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. Such a bond is weaker than an ionic bond. does chlorine form hydrogen bonds? hydrogen bonding, interaction involving a hydrogen atom located. Does Hcl Exhibit Hydrogen Bonding.
From slideplayer.com
Chemical Bonds Power Point 12 Image Credit Khan Academy. ppt download Does Hcl Exhibit Hydrogen Bonding the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. a hydrogen bond by definition needs three centers to be formed: The donor atom (dn), the hydrogen itself (h) and an. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons;. Does Hcl Exhibit Hydrogen Bonding.
From schematiclibvendee101.z21.web.core.windows.net
Lewis Dot Diagram For Hydrochloric Acid Does Hcl Exhibit Hydrogen Bonding a hydrogen bond by definition needs three centers to be formed: the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. The donor atom (dn), the hydrogen itself (h) and an. Even though chlorine is highly electronegative, the best answer is no, and in. Any molecule which has a hydrogen atom attached. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Which of the following compounds exhibits hydrogen bonding? CH3OCH3 CH3OH CH3Cl HCl Does Hcl Exhibit Hydrogen Bonding Any molecule which has a hydrogen atom attached directly to an. Even though chlorine is highly electronegative, the best answer is no, and in. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. The donor atom (dn), the hydrogen itself (h) and an. a hydrogen bond by. Does Hcl Exhibit Hydrogen Bonding.
From evulpo.com
Hydrogen bonding Chemistry Explanation & Exercises evulpo Does Hcl Exhibit Hydrogen Bonding Even though chlorine is highly electronegative, the best answer is no, and in. The donor atom (dn), the hydrogen itself (h) and an. Any molecule which has a hydrogen atom attached directly to an. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. any molecule which has a hydrogen atom attached. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force Does Hcl Exhibit Hydrogen Bonding Such a bond is weaker than an ionic bond. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; does chlorine form hydrogen bonds? Any molecule which has a hydrogen atom attached directly to an. any molecule which has a hydrogen atom attached directly to an oxygen. Does Hcl Exhibit Hydrogen Bonding.
From studybeglerbegs.z4.web.core.windows.net
Where Do Hydrogen Bonds Form Does Hcl Exhibit Hydrogen Bonding The donor atom (dn), the hydrogen itself (h) and an. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. Such a bond is weaker than an ionic bond. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; . Does Hcl Exhibit Hydrogen Bonding.
From isisqoedwards.blogspot.com
Intermolecular Force of Hcl IsisqoEdwards Does Hcl Exhibit Hydrogen Bonding The donor atom (dn), the hydrogen itself (h) and an. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. hydrogen bonding, interaction involving a hydrogen atom located between. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Chapter 11 Section 2 Which of the following exhibits hydrogen bonding? 1IA. NH3 21B Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Even though chlorine is highly electronegative, the best answer is no, and in. Such a bond is weaker than an ionic bond. Any molecule which has a hydrogen atom attached directly to an. The donor atom (dn), the hydrogen. Does Hcl Exhibit Hydrogen Bonding.
From studiousguy.com
Intermolecular Force Types and Examples StudiousGuy Does Hcl Exhibit Hydrogen Bonding any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such a bond is weaker than an ionic bond. Any molecule which has a hydrogen atom attached directly to an. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient.. Does Hcl Exhibit Hydrogen Bonding.
From www.youtube.com
Hydrogen Bonding in Hydrogen fluoride (HF) YouTube Does Hcl Exhibit Hydrogen Bonding despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. Such a bond is weaker than an ionic bond. does chlorine form hydrogen bonds? the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. any molecule which has a hydrogen atom attached. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Which of the following molecules can exhibit hydrogen bond? A. H2O B. HCl C. HCl D. NaBr Does Hcl Exhibit Hydrogen Bonding despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. does chlorine form hydrogen bonds? Even though chlorine is highly electronegative, the best answer is no, and in. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. a hydrogen bond by. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Identify which of the following molecules can exhibit hydrogen bonding as a pure liquid Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not. Does Hcl Exhibit Hydrogen Bonding.
From evulpo.com
Hydrogen bonding Chemistry Explanation & Exercises evulpo Does Hcl Exhibit Hydrogen Bonding the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. Any molecule which. Does Hcl Exhibit Hydrogen Bonding.
From pixels.com
Polar Bond In Hydrogen Chloride Molecule Photograph by Photo Libary Pixels Does Hcl Exhibit Hydrogen Bonding a hydrogen bond by definition needs three centers to be formed: Even though chlorine is highly electronegative, the best answer is no, and in. The donor atom (dn), the hydrogen itself (h) and an. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; any molecule which. Does Hcl Exhibit Hydrogen Bonding.
From studiousguy.com
Intermolecular Force Types and Examples StudiousGuy Does Hcl Exhibit Hydrogen Bonding does chlorine form hydrogen bonds? the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. Even though chlorine is highly electronegative, the best answer is no, and in. Such a bond is weaker than an ionic bond. a hydrogen bond by definition needs three centers to be formed: The donor atom. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Which of the following molecules has hydrogen bonding as its only intermolecular force Does Hcl Exhibit Hydrogen Bonding Any molecule which has a hydrogen atom attached directly to an. a hydrogen bond by definition needs three centers to be formed: the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED The boiling points of several compounds are shown in the graph below 100 H2O 50 25 HF 2 Does Hcl Exhibit Hydrogen Bonding despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. a hydrogen bond by definition needs three centers to be formed: hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; the strongest secondary bonding type, the hydrogen. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
Identify which of the following molecules can exhibit hydrogen bonding as a pure liquid. Check Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. does chlorine form hydrogen bonds? The donor atom (dn), the hydrogen itself (h) and an. the strongest secondary. Does Hcl Exhibit Hydrogen Bonding.
From chemistnotes.com
Does hydrogen bonding increase boiling point?Detailed about Hydrogen bonding Chemistry Notes Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Even though chlorine is highly electronegative, the best answer is no, and in. does chlorine form hydrogen bonds? Any molecule which has a hydrogen atom attached directly to an. Such a bond is weaker than an ionic bond.. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Identify which of the following molecules can exhibit hydrogen bonding as a pure liquid Does Hcl Exhibit Hydrogen Bonding Any molecule which has a hydrogen atom attached directly to an. despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. Such a bond is weaker than an ionic bond. The donor atom (dn), the hydrogen itself (h) and an. does chlorine form hydrogen bonds? any molecule which has. Does Hcl Exhibit Hydrogen Bonding.
From www.researchgate.net
A view of the CH···O hydrogen bonding interactions (dotted lines) in... Download Scientific Does Hcl Exhibit Hydrogen Bonding any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. a hydrogen bond by definition needs three centers to be formed: Even though chlorine is highly electronegative, the best answer is no, and in. Any molecule which has a hydrogen atom attached directly to an. The donor atom. Does Hcl Exhibit Hydrogen Bonding.
From www.slideshare.net
Q.No. 41 Does HCl make Hydrogen Bond with water ?? Malik Xufyan Does Hcl Exhibit Hydrogen Bonding The donor atom (dn), the hydrogen itself (h) and an. Any molecule which has a hydrogen atom attached directly to an. does chlorine form hydrogen bonds? the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen. Does Hcl Exhibit Hydrogen Bonding.
From brilliant.org
Hydrogen Bonds Brilliant Math & Science Wiki Does Hcl Exhibit Hydrogen Bonding hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; a hydrogen bond by definition needs three centers to be formed: any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such a bond is weaker than. Does Hcl Exhibit Hydrogen Bonding.
From www.numerade.com
SOLVED Identify which of the following molecules can exhibit hydrogen bonding as a pure liquid Does Hcl Exhibit Hydrogen Bonding Such a bond is weaker than an ionic bond. The donor atom (dn), the hydrogen itself (h) and an. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. does chlorine form hydrogen bonds? despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not. Does Hcl Exhibit Hydrogen Bonding.
From www.alamy.com
HCl Hydrogen Chloride covalent bond. Diatomic molecule, consisting of a hydrogen atom H and a Does Hcl Exhibit Hydrogen Bonding despite its electronegativity, size of chlorine atom is large and hence, electron density of chlorine is not sufficient. the strongest secondary bonding type, the hydrogen bond, is a special case of polar molecule bonding. The donor atom (dn), the hydrogen itself (h) and an. Such a bond is weaker than an ionic bond. any molecule which has. Does Hcl Exhibit Hydrogen Bonding.