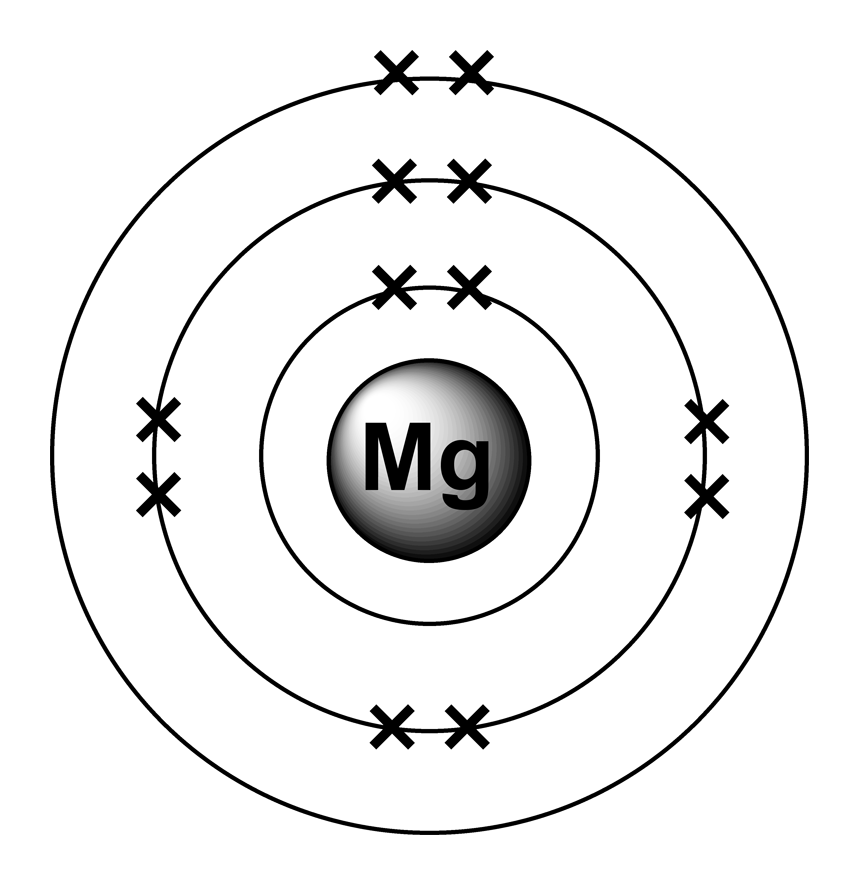
Does Magnesium Give Or Take Electrons . (b) since n 3− is an anion, its. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. There are, however, a small number of. A magnesium ion therefore has 10 electrons. To get a +2 charge, the ion had to lose two electrons. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. Hydrogen is an exception, as it will usually. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion.
from www.animalia-life.club
In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually. (b) since n 3− is an anion, its. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. A magnesium ion therefore has 10 electrons. To get a +2 charge, the ion had to lose two electrons. There are, however, a small number of.
Magnesium Electron Configuration
Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. To get a +2 charge, the ion had to lose two electrons. (b) since n 3− is an anion, its. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. There are, however, a small number of. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. Hydrogen is an exception, as it will usually. A magnesium ion therefore has 10 electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools.
From www.shutterstock.com
Diagram Representation Element Magnesium Neutrons Protons Stock Vector Does Magnesium Give Or Take Electrons A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Hydrogen is an exception, as it will usually. There are, however, a small number of. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. (b) since. Does Magnesium Give Or Take Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. (b) since n 3− is an anion, its. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Some atoms have. Does Magnesium Give Or Take Electrons.
From www.alamy.com
Atom Symbol for Magnesium Stock Vector Image & Art Alamy Does Magnesium Give Or Take Electrons (b) since n 3− is an anion, its. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. To get a +2 charge, the ion had to lose two electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative. Does Magnesium Give Or Take Electrons.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Does Magnesium Give Or Take Electrons To get a +2 charge, the ion had to lose two electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. (b) since n 3− is an anion,. Does Magnesium Give Or Take Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually. There are,. Does Magnesium Give Or Take Electrons.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Does Magnesium Give Or Take Electrons In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. A magnesium ion therefore has 10 electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. (b) since n 3− is an anion, its. A magnesium atom. Does Magnesium Give Or Take Electrons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Does Magnesium Give Or Take Electrons Hydrogen is an exception, as it will usually. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. To get a +2 charge, the ion had to lose two electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have. Does Magnesium Give Or Take Electrons.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Does Magnesium Give Or Take Electrons There are, however, a small number of. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. (b) since n 3− is an anion, its. Hydrogen is an exception, as it will usually. To get a +2 charge, the ion had to lose two electrons. In general, metals. Does Magnesium Give Or Take Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Does Magnesium Give Or Take Electrons There are, however, a small number of. (b) since n 3− is an anion, its. To get a +2 charge, the ion had to lose two electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. A magnesium atom must lose two electrons to have the same number. Does Magnesium Give Or Take Electrons.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. To get a +2 charge, the ion had to lose two electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. (b) since n 3− is an anion, its. In general, metals will lose electrons to become a positive cation and. Does Magnesium Give Or Take Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. A magnesium atom must lose two electrons to have the same number. Does Magnesium Give Or Take Electrons.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Does Magnesium Give Or Take Electrons A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. To get a +2 charge, the ion had to lose two electrons. Hydrogen is an exception, as it will usually. (b) since n 3− is an anion, its. In compounds, magnesium virtually always exhibits a +2 oxidation state. Does Magnesium Give Or Take Electrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Does Magnesium Give Or Take Electrons There are, however, a small number of. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. In general, metals will lose electrons to become a positive. Does Magnesium Give Or Take Electrons.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Does Magnesium Give Or Take Electrons A magnesium ion therefore has 10 electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Hydrogen is an exception, as it will usually. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. There are,. Does Magnesium Give Or Take Electrons.
From www.youtube.com
Valence Electrons for Magnesium (Mg) YouTube Does Magnesium Give Or Take Electrons Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. (b) since n 3− is an anion, its. There are, however, a small number of. A magnesium ion therefore. Does Magnesium Give Or Take Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Does Magnesium Give Or Take Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. There are, however, a small number of. (b) since n 3− is an anion, its. To get a +2 charge, the ion had to lose two electrons. Hydrogen is an exception, as it will usually. A magnesium atom must. Does Magnesium Give Or Take Electrons.
From nursehub.com
Electron Shells NurseHub Does Magnesium Give Or Take Electrons A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Hydrogen is an exception, as it will usually. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. To get a +2 charge, the ion had to. Does Magnesium Give Or Take Electrons.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Does Magnesium Give Or Take Electrons In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. Hydrogen is an exception, as it will usually. (b) since n 3− is an anion, its. To get a +2 charge, the ion had to lose two electrons. There are, however, a small number of. Magnesium is used in. Does Magnesium Give Or Take Electrons.
From www.youtube.com
Magnesium Electron Configuration YouTube Does Magnesium Give Or Take Electrons In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. There are, however, a small number of. Hydrogen is an exception, as it will usually. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. (b) since n. Does Magnesium Give Or Take Electrons.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Does Magnesium Give Or Take Electrons (b) since n 3− is an anion, its. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Hydrogen is an exception, as it will usually. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. In general, metals. Does Magnesium Give Or Take Electrons.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Does Magnesium Give Or Take Electrons To get a +2 charge, the ion had to lose two electrons. (b) since n 3− is an anion, its. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Does Magnesium Give Or Take Electrons.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Does Magnesium Give Or Take Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. A magnesium ion therefore has 10 electrons. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. (b) since n 3− is an anion, its. There are, however,. Does Magnesium Give Or Take Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Does Magnesium Give Or Take Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. To get a +2 charge, the ion had to lose two electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is used in products. Does Magnesium Give Or Take Electrons.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Does Magnesium Give Or Take Electrons To get a +2 charge, the ion had to lose two electrons. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. A magnesium atom must lose two. Does Magnesium Give Or Take Electrons.
From www.youtube.com
How many valence electrons does magnesium have? YouTube Does Magnesium Give Or Take Electrons There are, however, a small number of. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. To get a +2 charge, the ion had to lose two electrons. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons.. Does Magnesium Give Or Take Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Does Magnesium Give Or Take Electrons To get a +2 charge, the ion had to lose two electrons. A magnesium ion therefore has 10 electrons. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. There are, however, a small number of. A magnesium atom must lose two electrons to have the same number electrons as. Does Magnesium Give Or Take Electrons.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Does Magnesium Give Or Take Electrons A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of. Does Magnesium Give Or Take Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5581477 Does Magnesium Give Or Take Electrons There are, however, a small number of. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. A magnesium ion therefore has 10 electrons. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. In compounds, magnesium virtually. Does Magnesium Give Or Take Electrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Does Magnesium Give Or Take Electrons Hydrogen is an exception, as it will usually. (b) since n 3− is an anion, its. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. A. Does Magnesium Give Or Take Electrons.
From www.slideserve.com
PPT How many valence electrons does magnesium have? PowerPoint Does Magnesium Give Or Take Electrons Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. (b) since n 3− is an anion, its. A magnesium ion therefore has 10 electrons. Hydrogen is an. Does Magnesium Give Or Take Electrons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Does Magnesium Give Or Take Electrons Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. A magnesium ion therefore has 10 electrons. To get a +2 charge, the ion had to lose two electrons. There are, however, a small number of. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss. Does Magnesium Give Or Take Electrons.
From www.youtube.com
What is the electron configuration of magnesium, Mg? YouTube Does Magnesium Give Or Take Electrons A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. (b) since n 3− is an anion, its. Some atoms have nearly eight electrons in their valence. Does Magnesium Give Or Take Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Does Magnesium Give Or Take Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Hydrogen is an exception, as it will usually. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. (b) since n 3− is an anion, its. Magnesium. Does Magnesium Give Or Take Electrons.
From www.alamy.com
Symbol and electron diagram for Magnesium illustration Stock Vector Does Magnesium Give Or Take Electrons Hydrogen is an exception, as it will usually. (b) since n 3− is an anion, its. To get a +2 charge, the ion had to lose two electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Some atoms have nearly eight electrons in their valence shell. Does Magnesium Give Or Take Electrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Does Magnesium Give Or Take Electrons To get a +2 charge, the ion had to lose two electrons. There are, however, a small number of. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an. Does Magnesium Give Or Take Electrons.