Raw Material Sampling Guidelines . Who guidelines for sampling of pharmaceutical products and related materials. This article discusses reduced sampling and testing of starting materials or components. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for.
from www.inpaspages.com
Who guidelines for sampling of pharmaceutical products and related materials. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. This article discusses reduced sampling and testing of starting materials or components.
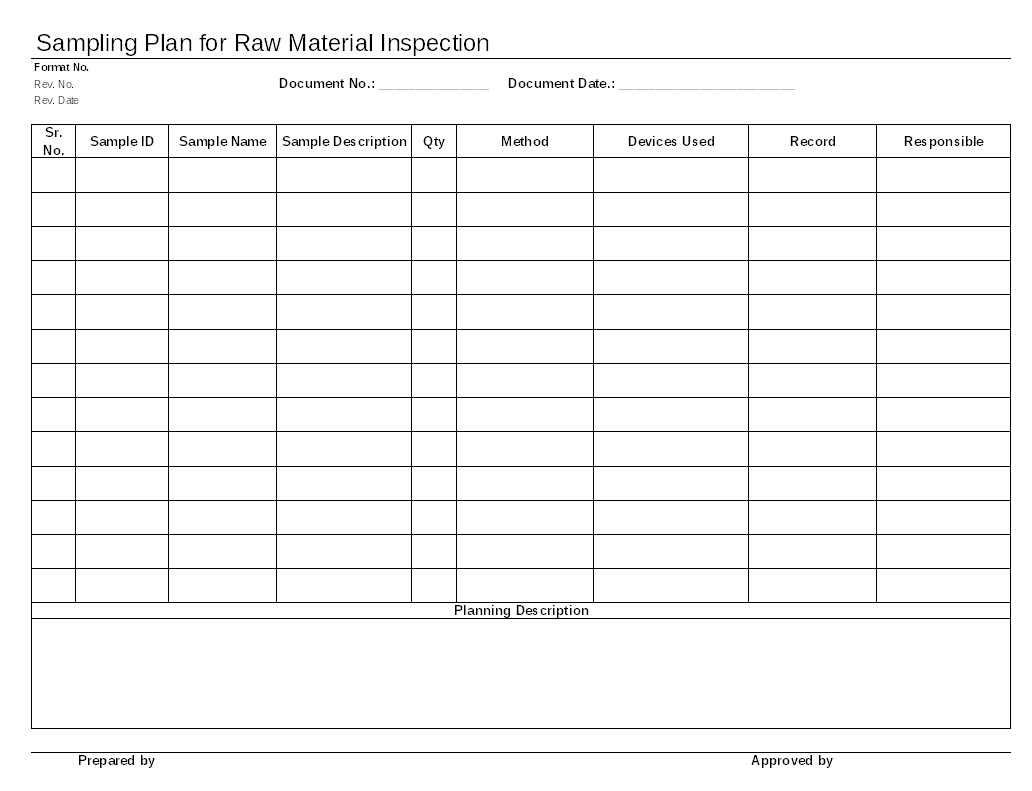
Documentation of sampling plan for raw material inspection
Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled.
From www.researchgate.net
(PDF) Sampling Procedures to Determine the Proportion of Raw Material Sampling Guidelines This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be. Raw Material Sampling Guidelines.
From www.slideshare.net
SAMPLING METHODS Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling procedure should be appropriate to the purpose of sampling, to the. Raw Material Sampling Guidelines.
From www.slideserve.com
PPT Implement sampling procedures PowerPoint Presentation, free Raw Material Sampling Guidelines Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Who guidelines for sampling of pharmaceutical products and related materials. The sampling procedure should be appropriate to the purpose of sampling, to. Raw Material Sampling Guidelines.
From www.laafon.com
SOP for sampling of Raw Materials Laafon Galaxy Pharmaceuticals Raw Material Sampling Guidelines Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. This article discusses reduced sampling and testing of starting materials or components. The. Raw Material Sampling Guidelines.
From www.academia.edu
(PDF) Raw Material Specification Template Narongchai Pongpan Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access. Raw Material Sampling Guidelines.
From www.pharmaqualification.com
Hold Time Study Raw Material Sampling Guidelines These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Who guidelines for sampling of pharmaceutical products and related materials. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. Access. Raw Material Sampling Guidelines.
From www.scribd.com
Sampling Plan of Raw Materials PDF Raw Material Sampling Guidelines This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be. Raw Material Sampling Guidelines.
From www.studentsassignmenthelp.com
Sampling Method Types along with example of selecting a sample Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries. Raw Material Sampling Guidelines.
From ethidelabs.com
Ethide Laboratories Sampling For Sterility Testing Raw Material Sampling Guidelines Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. This article discusses reduced sampling and. Raw Material Sampling Guidelines.
From favouritehub.com
Know more About Sanitary pad raw material details Sanitary Pad Raw Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access. Raw Material Sampling Guidelines.
From www.researchgate.net
Prepare Raw Material, sampling process and characterizations Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices. Raw Material Sampling Guidelines.
From bulksale.jeevaorganic.com
Sourcing Raw Ingredients for Manufacturing Vitamin Supplement Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be. Raw Material Sampling Guidelines.
From ezine-articles.com
Celery Juice Processing Plant Project Report 2024 Cost Analysis and Raw Material Sampling Guidelines Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. Access to medicines and health products (mhp), health product policy and standards (hps),. Raw Material Sampling Guidelines.
From www.youtube.com
Raw Material Sampling YouTube Raw Material Sampling Guidelines These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure (sop) for sampling of raw material (api & excipient) as. Raw Material Sampling Guidelines.
From www.quality-assurance-solutions.com
AQL Sampling Plans, Step by Step understanding of AQL Raw Material Sampling Guidelines This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Access to medicines and health products (mhp), health product policy. Raw Material Sampling Guidelines.
From mypharmasops.blogspot.com
SOP's For (Pharmaceuticals) Flow Chart of Raw Material Store Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure (sop) for sampling of raw material (api & excipient). Raw Material Sampling Guidelines.
From dglabservices.com
Sampling Systems DG Lab Services Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Access to medicines and health products (mhp), health product policy and standards. Raw Material Sampling Guidelines.
From pharmablog.in
Sampling, Testing and Approval/Rejection of Packaging Material SOP Raw Material Sampling Guidelines This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be. Raw Material Sampling Guidelines.
From tech-publish.com
SOP For Sampling Of Raw Materials In Pharmaceuticals Techpublish Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to. Raw Material Sampling Guidelines.
From mungfali.com
Inspection Process Flow Chart Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Who guidelines for sampling of pharmaceutical products and related materials. This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling. Raw Material Sampling Guidelines.
From www.inpaspages.com
Raw material control process Raw Material Sampling Guidelines The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. Access to. Raw Material Sampling Guidelines.
From wespec.net
SPEC OF RAW MATERIALS WESPEC Raw Material Sampling Guidelines This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling procedure should be appropriate to the purpose of. Raw Material Sampling Guidelines.
From www.mdpi.com
Polymers Free FullText Sampling Scheme Conception for Pretreated Raw Material Sampling Guidelines These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. Access. Raw Material Sampling Guidelines.
From www.youtube.com
Sampling of Raw Materials YouTube Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Access to medicines and health products (mhp), health product policy and standards. Raw Material Sampling Guidelines.
From researz.com
Detailed Report on Leatherette Manufacturing Plant Setup Cost, Layout Raw Material Sampling Guidelines The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. These guidelines. Raw Material Sampling Guidelines.
From www.complianceonline.com
CGMP controlled Raw Materials Regulations and Best Practices Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. This article discusses reduced sampling and testing of starting materials or components. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling procedure should be appropriate to the purpose of sampling, to. Raw Material Sampling Guidelines.
From www.inpaspages.com
Documentation of sampling plan for raw material inspection Raw Material Sampling Guidelines Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule. Raw Material Sampling Guidelines.
From pharmablog.in
Sampling of Raw material and cleaning of sampling aidsSOP PharmaBlog Raw Material Sampling Guidelines These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in. Raw Material Sampling Guidelines.
From www.aanandsingh.com.np
SOP for Sampling of Raw Material in Pharmaceutical Industry Raw Material Sampling Guidelines Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Who guidelines for sampling of pharmaceutical products and related materials. This article discusses reduced sampling and testing of starting materials or components. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. The. Raw Material Sampling Guidelines.
From pharmablog.in
Sampling of Raw material and cleaning of sampling aidsSOP PharmaBlog Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. The sampling procedure should be appropriate to the purpose of sampling, to the. Raw Material Sampling Guidelines.
From pharmablog.in
Analysis of Raw Materials SOP PharmaBlog Raw Material Sampling Guidelines Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples. Raw Material Sampling Guidelines.
From www.scribd.com
SOP for Sampling and Release of Raw Materials _ Pharmaceutical Raw Material Sampling Guidelines The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to the samples and to the material to be sampled. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule m and ich q7. Who guidelines for sampling of pharmaceutical products and related materials. These. Raw Material Sampling Guidelines.
From pharmaguidelines.co.uk
Standard Operating Procedure (SOP) for Raw Material Sampling and Raw Material Sampling Guidelines These guidelines are consistent with the requirements of the who guidelines for good manufacturing practices (1) and with the. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. This article discusses reduced sampling and testing of starting materials or components. Access to medicines and health products (mhp), health product policy and. Raw Material Sampling Guidelines.
From www.slideshare.net
Raw material Raw Material Sampling Guidelines Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. This article discusses reduced sampling and testing of starting materials or components. Who guidelines for sampling of pharmaceutical products and related materials. The sampling procedure should be appropriate to the purpose of sampling, to the type of controls intended to be applied to. Raw Material Sampling Guidelines.
From www.researchgate.net
Prepare Raw Material, sampling process and characterizations Raw Material Sampling Guidelines Who guidelines for sampling of pharmaceutical products and related materials. Standard operating procedure of sampling of raw material received in stores in pharmaceutical industries using square root of. Access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. Standard operating procedure (sop) for sampling of raw material (api & excipient) as per schedule. Raw Material Sampling Guidelines.