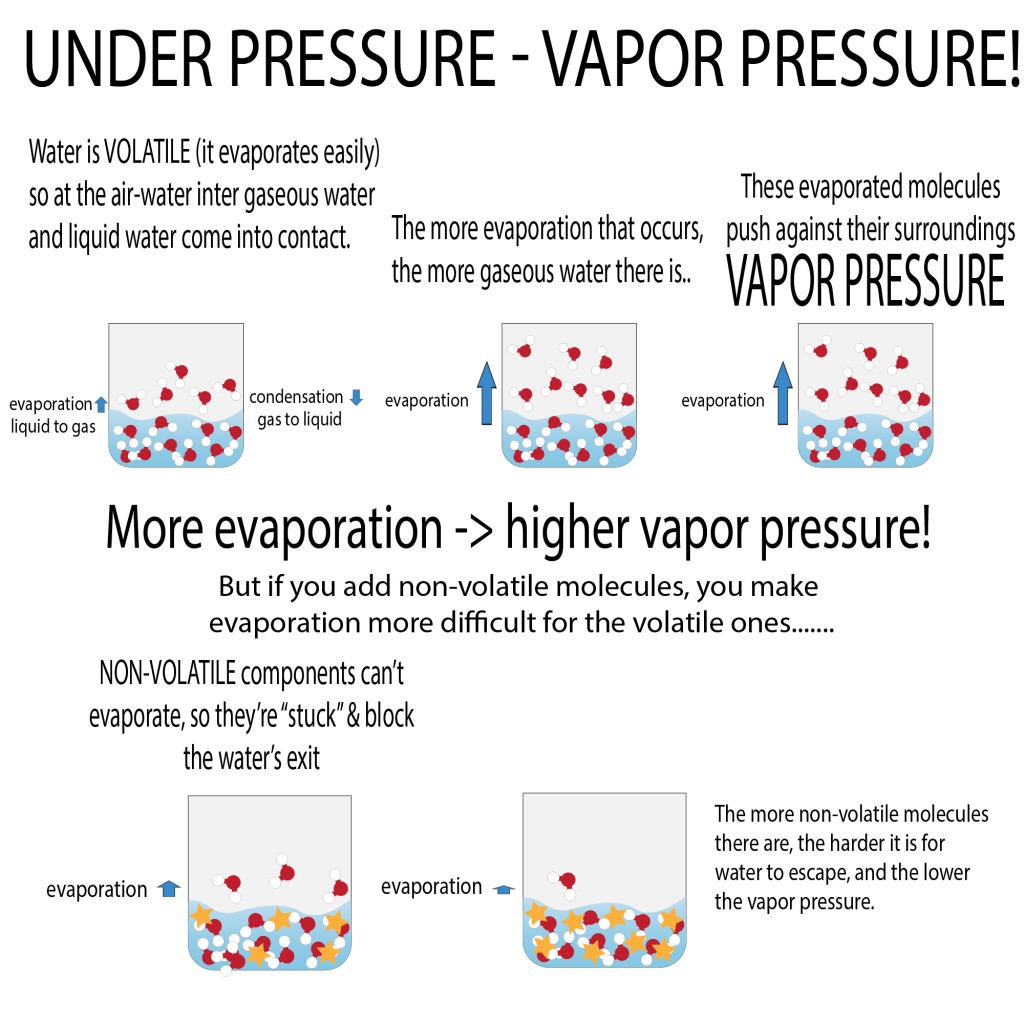
Will Vapor Pressure Increase With Temperature . Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. We would expect it to get larger as the temperature is raised. The increase in vapor pressure with temperature is not linear, however. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. How does the vapor pressure change with temperature? To understand that the relationship between pressure,. The line on the graph shows the boiling.
from inspiritvr.com
We would expect it to get larger as the temperature is raised. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The line on the graph shows the boiling. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the relationship between pressure,. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. How does the vapor pressure change with temperature? The increase in vapor pressure with temperature is not linear, however. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present.
Vapor Pressure Lowering Study Guide Inspirit
Will Vapor Pressure Increase With Temperature Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. How does the vapor pressure change with temperature? The line on the graph shows the boiling. To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. We would expect it to get larger as the temperature is raised. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Will Vapor Pressure Increase With Temperature The line on the graph shows the boiling. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the relationship between pressure,. How does the vapor pressure change with temperature? For example, consider the vapor pressure of water between 0°c and. Will Vapor Pressure Increase With Temperature.
From www.nuclear-power.com
Temperatureentropy Diagrams Ts Diagrams Will Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? To understand that the relationship between pressure,. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. We would expect it to get larger as the temperature is raised. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and. Will Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved (4pts) The graph below is the vapor pressures of Will Vapor Pressure Increase With Temperature The line on the graph shows the boiling. How does the vapor pressure change with temperature? The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressure is the pressure exerted by a. Will Vapor Pressure Increase With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Will Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. To understand that the relationship between pressure,. We would expect it to get larger as the temperature is raised. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a. Will Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved Over a water surface the saturation vapor pressure es Will Vapor Pressure Increase With Temperature The line on the graph shows the boiling. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. To understand that the relationship between pressure,. We would expect it to get larger as the. Will Vapor Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The increase in vapor pressure with temperature is not linear, however. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. For example, consider the. Will Vapor Pressure Increase With Temperature.
From www.researchgate.net
Correlation between temperature and saturation vapor pressure [4 Will Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The increase in vapor pressure with temperature is not linear,. Will Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED The vapor pressure of methanol is 0.128 atm at 20.0 ºC. The Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. How does the vapor pressure change with temperature? The line on the graph shows the boiling. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system. Will Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED does vapor pressure increase or decrease with temperature Will Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature is raised. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and. Will Vapor Pressure Increase With Temperature.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. We would expect it to get larger as the temperature is raised. Vapor pressure is the pressure. Will Vapor Pressure Increase With Temperature.
From brainly.com
At which temperature is the vapor pressure of ethanol equal to 80. kPa Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. Vapor pressure is the pressure exerted. Will Vapor Pressure Increase With Temperature.
From techblog.ctgclean.com
Drying Vapor Pressure vs. Temperature CTG Clean Will Vapor Pressure Increase With Temperature The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. We would expect it to get larger as the temperature is raised. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the equilibrium. Will Vapor Pressure Increase With Temperature.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit Will Vapor Pressure Increase With Temperature Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the relationship between pressure,. The increase in vapor pressure with temperature is not linear, however. The vapor pressure of a liquid varies with its temperature, as the following graph shows for. Will Vapor Pressure Increase With Temperature.
From www.vrogue.co
What Is A Temperature Where The Vapor Pressure Of A L vrogue.co Will Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature is raised. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. How does the vapor pressure change with temperature? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressure is. Will Vapor Pressure Increase With Temperature.
From mungfali.com
Water Vapor Pressure Temperature Chart Will Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the relationship between pressure,. We would expect it to get larger as the. Will Vapor Pressure Increase With Temperature.
From www.indium.com
Properties of Gallium & Gallium Alloys Indium Corporation Will Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they. Will Vapor Pressure Increase With Temperature.
From www.chegg.com
Help me to find the answers from the top to bottom. Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The increase in vapor pressure with temperature is not linear, however. How does the vapor pressure change with temperature? We would expect it. Will Vapor Pressure Increase With Temperature.
From www.chemistrylearner.com
Saturated Vapor Pressure Definition and Temperature Effect Will Vapor Pressure Increase With Temperature The increase in vapor pressure with temperature is not linear, however. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid). Will Vapor Pressure Increase With Temperature.
From saylordotorg.github.io
Vapor Pressure Will Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature is raised. The increase in vapor pressure with temperature is not linear, however. The line on the graph shows the boiling. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. The vapor pressure. Will Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure as a function of temperature for carbon dioxide (CO 2 Will Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The line on the graph shows the boiling. The vapor pressure of a liquid varies with its temperature, as the following graph shows. Will Vapor Pressure Increase With Temperature.
From www.caee.utexas.edu
Vapor Pressure vs. Temperature Will Vapor Pressure Increase With Temperature The increase in vapor pressure with temperature is not linear, however. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The vapor pressure of. Will Vapor Pressure Increase With Temperature.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Will Vapor Pressure Increase With Temperature The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The increase in vapor pressure with temperature is not linear, however. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. How does the vapor pressure change with temperature? For example, consider the vapor. Will Vapor Pressure Increase With Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Will Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? The increase in vapor pressure with temperature is not linear, however. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and. Will Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Will Vapor Pressure Increase With Temperature The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular. Will Vapor Pressure Increase With Temperature.
From mavink.com
Water Vapor Pressure Vs Temperature Will Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. We would expect it to get larger as the temperature is raised. How does the vapor pressure change with temperature? To understand that the equilibrium. Will Vapor Pressure Increase With Temperature.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Will Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. We would expect it to get larger as the temperature is raised. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. How does the vapor. Will Vapor Pressure Increase With Temperature.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Will Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The increase in vapor pressure with temperature is not linear, however. We would expect it to get larger. Will Vapor Pressure Increase With Temperature.
From vacaero.com
Partial Pressure Brazing Will Vapor Pressure Increase With Temperature The line on the graph shows the boiling. We would expect it to get larger as the temperature is raised. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. To understand that the relationship between pressure,. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when. Will Vapor Pressure Increase With Temperature.
From mavink.com
Water Vapour Pressure Chart Bar Will Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature is raised. The line on the graph shows the boiling. The increase in vapor pressure with temperature is not linear, however. To understand that the relationship between pressure,. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. To understand that the equilibrium. Will Vapor Pressure Increase With Temperature.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Will Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. How does the vapor pressure change with temperature? The increase in vapor pressure with temperature is not linear, however. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. We would expect it to get larger as. Will Vapor Pressure Increase With Temperature.
From mrlvohyvol.blogspot.com
How To Find Vapor Pressure Of A Solution Determine vapor pressure of Will Vapor Pressure Increase With Temperature The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The increase in vapor pressure with temperature is not linear, however. How does the vapor pressure change with temperature? The line on the graph shows the boiling. To understand that the relationship between pressure,. We would expect it to get larger as the. Will Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved Shown below is the vapor pressure curve for water Will Vapor Pressure Increase With Temperature Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. To understand that the relationship. Will Vapor Pressure Increase With Temperature.
From mungfali.com
CO2 Pressure Temperature Chart Will Vapor Pressure Increase With Temperature The increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. We would expect it to get larger as the temperature is raised. To understand that the relationship between pressure,. The vapor pressure of a liquid varies with its temperature, as the. Will Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure as a function of temperature. The trend lines are the Will Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The increase in vapor pressure with temperature is not linear, however. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. We would expect it to. Will Vapor Pressure Increase With Temperature.
From courses.lumenlearning.com
Liquid to Gas Phase Transition Introduction to Chemistry Will Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in figure \(\pageindex{1}\) below. The line on the graph shows the boiling. To understand that the relationship between pressure,. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. How. Will Vapor Pressure Increase With Temperature.