Zinc Hydroxide And Ammonia Reaction . reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. Zinc(ii) ion forms complex ions readily. zinc chloride (zncl2) reacts with ammonia and produce a white colour precipitate zinc hydroxide ( zn(oh)2 ). Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. testing these ions using ammonia solution. Zinc(ii) ion reacts with aqueous. Addition of aqueous ammonia to the zn 2+ aqueous solution. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. — i understand zink can react with an acid or a base to form different compounds in solution based on ph. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble in acids. Ammonia reacts with water to some extent to form ammonium hydroxide.
from www.teachoo.com
reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. zinc +2 ion and dilute ammonia solution reaction. — i understand zink can react with an acid or a base to form different compounds in solution based on ph. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. — aqueous ammonia. Zinc(ii) ion forms complex ions readily. One mole of zinc sulfate [znso 4], two moles of. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:.
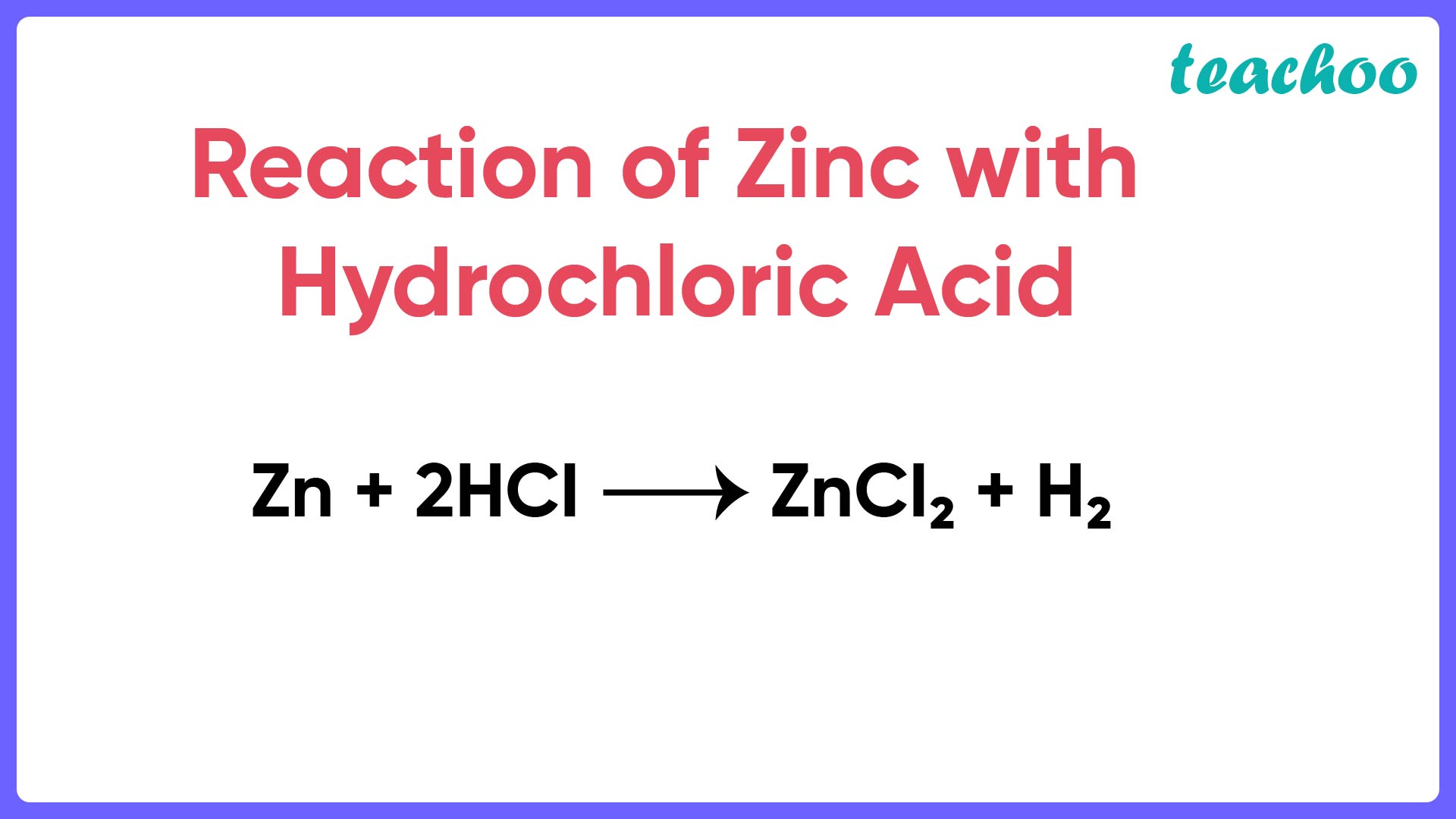
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro
Zinc Hydroxide And Ammonia Reaction for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble in acids. reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. — aqueous ammonia. — this paper focuses on the efficient extraction of valuable metal zinc from szo dust by proposing a process for. Zn(oh) 2 (s) + 4. In this experiment, students add ammonia to a. One mole of zinc sulfate [znso 4], two moles of. zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. zinc +2 ion and dilute ammonia solution reaction. Addition of aqueous ammonia to the zn 2+ aqueous solution. testing these ions using ammonia solution. When aqueous dilute ammonia solution is added slowly to the colourless. Zinc metal (zn) reacts with ammonium hydroxide, which is a weak base. for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:.
From brainly.in
zinc is put in concentrated sodium hydroxide. explain with the help of Zinc Hydroxide And Ammonia Reaction Zinc(ii) ion reacts with aqueous. — i understand zink can react with an acid or a base to form different compounds in solution based on ph. Ammonia reacts with water to some extent to form ammonium hydroxide. Zn(oh) 2 (s) + 4. Addition of aqueous ammonia to the zn 2+ aqueous solution. zinc +2 ion and dilute ammonia. Zinc Hydroxide And Ammonia Reaction.
From www.nagwa.com
Question Video Recalling the Observed Products of Reactions of Metal Zinc Hydroxide And Ammonia Reaction — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble. Zinc Hydroxide And Ammonia Reaction.
From zelengarden.ru
Закончите схему реакции zn h2so4 конц s Zinc Hydroxide And Ammonia Reaction One mole of zinc sulfate [znso 4], two moles of. try this class practical to explore an equilibrium involving copper (ii) ions. Zinc(ii) ion forms complex ions readily. In this experiment, students add ammonia to a. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. — this review focuses on. Zinc Hydroxide And Ammonia Reaction.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Zinc Hydroxide And Ammonia Reaction Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. try this class practical to explore an equilibrium involving copper (ii) ions. — as an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of. Zinc Hydroxide And Ammonia Reaction.
From www.youtube.com
Test for Zinc ion with Aqueous Ammonia YouTube Zinc Hydroxide And Ammonia Reaction — i understand zink can react with an acid or a base to form different compounds in solution based on ph. In this experiment, students add ammonia to a. testing these ions using ammonia solution. — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zn(oh) 2 (s) + 4.. Zinc Hydroxide And Ammonia Reaction.
From www.researchgate.net
Schematic representation of antibacterial mechanism of zinc oxide Zinc Hydroxide And Ammonia Reaction — aqueous ammonia. Zn(oh) 2 (s) + 4. Nh 3 (aq) + h 2 o (l) ? characteristic reactions of zn 2+: — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. try this class practical to explore an equilibrium involving copper (ii) ions. reaction. Zinc Hydroxide And Ammonia Reaction.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Hydroxide And Ammonia Reaction — as an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Addition of aqueous ammonia to the zn 2+ aqueous solution. reaction with ammonium hydroxide. reaction of aqueous ammonia and zinc ion solution | nh 3. Zinc Hydroxide And Ammonia Reaction.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Zinc Hydroxide And Ammonia Reaction Addition of aqueous ammonia to the zn 2+ aqueous solution. zinc chloride (zncl2) reacts with ammonia and produce a white colour precipitate zinc hydroxide ( zn(oh)2 ). — as an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6]. Zinc Hydroxide And Ammonia Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Hydroxide And Ammonia Reaction zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble in acids. Addition of aqueous ammonia to the zn 2+ aqueous solution. Zn(oh) 2 (s) + 4. Nh 3 (aq) + h 2 o (l) ? — as an example of the formation of. Zinc Hydroxide And Ammonia Reaction.
From www.researchgate.net
(PDF) Reaction of zinc oxide with ammonium chloride Zinc Hydroxide And Ammonia Reaction zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. Nh 3 (aq) + h 2 o (l) ? One mole of zinc sulfate [znso 4], two moles of. Zn(oh) 2 (s) + 4. Zinc metal (zn) reacts with ammonium hydroxide, which is a weak base. — i understand zink can react with an acid or. Zinc Hydroxide And Ammonia Reaction.
From brainly.in
Reaction of zinc with water Brainly.in Zinc Hydroxide And Ammonia Reaction Nh 3 (aq) + h 2 o (l) ? — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. — this paper focuses on the efficient extraction of valuable metal zinc from szo dust by proposing a process for. — the zinc ion will react with the ammonia solution to. Zinc Hydroxide And Ammonia Reaction.
From ar.inspiredpencil.com
Ammonia Gas Test Zinc Hydroxide And Ammonia Reaction — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. Zn(oh) 2 (s). Zinc Hydroxide And Ammonia Reaction.
From www.doubtnut.com
[Telugu] How is ammonia manufactured by Haber's process ? Explain the Zinc Hydroxide And Ammonia Reaction zinc +2 ion and dilute ammonia solution reaction. Zn(oh) 2 (s) + 4. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. characteristic reactions of zn 2+: . Zinc Hydroxide And Ammonia Reaction.
From chemistry-europe.onlinelibrary.wiley.com
3D Flower‐Like Zinc Cobaltite for Electrocatalytic Reduction of Nitrate Zinc Hydroxide And Ammonia Reaction Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. In this experiment, students add ammonia to a. Ammonia reacts with water to some extent to form ammonium hydroxide. — i understand zink can react with an acid or a. Zinc Hydroxide And Ammonia Reaction.
From www.researchgate.net
Reversible reactions involved in the solgel method of zinc oxide Zinc Hydroxide And Ammonia Reaction Zinc(ii) ion forms complex ions readily. zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. Nh 3 (aq) + h 2 o (l) ? for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. Zn(oh) 2 (s) + 4. When aqueous dilute ammonia solution is. Zinc Hydroxide And Ammonia Reaction.
From www.youtube.com
Reaction of zinc sulphate (ZnSO4) with Ammonium hydroxide (NH4OH) YouTube Zinc Hydroxide And Ammonia Reaction Addition of aqueous ammonia to the zn 2+ aqueous solution. When aqueous dilute ammonia solution is added slowly to the colourless. reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. — as an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu. Zinc Hydroxide And Ammonia Reaction.
From www.nagwa.com
Question Video Selecting the Correction Equation for the Reversible Zinc Hydroxide And Ammonia Reaction zinc +2 ion and dilute ammonia solution reaction. try this class practical to explore an equilibrium involving copper (ii) ions. Zinc metal (zn) reacts with ammonium hydroxide, which is a weak base. When aqueous dilute ammonia solution is added slowly to the colourless. Addition of aqueous ammonia to the zn 2+ aqueous solution. — we propose a. Zinc Hydroxide And Ammonia Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for Fe(OH)3 + HNO3 = Fe(NO3)3 + H2O Zinc Hydroxide And Ammonia Reaction One mole of zinc sulfate [znso 4], two moles of. In this experiment, students add ammonia to a. — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. Addition of aqueous ammonia to the zn 2+ aqueous solution. Zn(oh) 2 (s) + 4. zinc chloride (zncl2) reacts with ammonia and produce. Zinc Hydroxide And Ammonia Reaction.
From www.numerade.com
SOLVED Ammonia burns in air (with a platinum catalyst), producing Zinc Hydroxide And Ammonia Reaction Zn(oh) 2 (s) + 4. characteristic reactions of zn 2+: — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. testing these ions using ammonia solution. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. Addition of aqueous. Zinc Hydroxide And Ammonia Reaction.
From brainly.in
2 ml of sodium hydroxide solution is added to a few pieces of Zinc Hydroxide And Ammonia Reaction testing these ions using ammonia solution. — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. the zinc hydroxide, in turn, reacts with the excess ammonia in the solution to form a colorless zinc ammonia complex, [zn(nh. Nh 3 (aq) + h 2 o (l) ? Zinc(ii) ion forms complex. Zinc Hydroxide And Ammonia Reaction.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Hydroxide And Ammonia Reaction reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. Addition of aqueous ammonia to the zn 2+ aqueous solution. zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. . Zinc Hydroxide And Ammonia Reaction.
From www.alamy.com
Labelled diagram to show the diffusion of ammonia and hydrogen Stock Zinc Hydroxide And Ammonia Reaction Zinc metal (zn) reacts with ammonium hydroxide, which is a weak base. Ammonia reacts with water to some extent to form ammonium hydroxide. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. zinc +2 ion and dilute ammonia solution reaction. zinc chloride (zncl2) reacts with ammonia and produce a white. Zinc Hydroxide And Ammonia Reaction.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Hydroxide And Ammonia Reaction zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. characteristic reactions of zn 2+: for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. —. Zinc Hydroxide And Ammonia Reaction.
From www.nagwa.com
Question Video Identifying a Metal Cation That Precipitates When a Few Zinc Hydroxide And Ammonia Reaction — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. reaction of aqueous. Zinc Hydroxide And Ammonia Reaction.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Salts Chemistry Revision & Tests Zinc Hydroxide And Ammonia Reaction — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. — as an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. — the zinc ion will react with the. Zinc Hydroxide And Ammonia Reaction.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The Zinc Hydroxide And Ammonia Reaction Ammonia reacts with water to some extent to form ammonium hydroxide. — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. Nh 3 (aq) + h 2 o (l) ? Zn(oh) 2 (s) + 4. Zinc(ii) ion reacts with aqueous. . Zinc Hydroxide And Ammonia Reaction.
From www.vecteezy.com
Preparation of ammonia in laboratory. ammonium chloride and calcium Zinc Hydroxide And Ammonia Reaction Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. In this experiment, students add ammonia to a. try this class practical to explore an equilibrium involving copper (ii) ions. One mole of zinc sulfate [znso 4], two moles of. — aqueous ammonia. characteristic reactions of zn 2+: zinc sulfate + ammonia + water =. Zinc Hydroxide And Ammonia Reaction.
From www.numerade.com
Ammonia gas NH3 and hydrogen sulphide gas H2S react together to form a Zinc Hydroxide And Ammonia Reaction reaction with ammonium hydroxide. Nh 3 (aq) + h 2 o (l) ? Zinc(ii) ion reacts with aqueous. — this paper focuses on the efficient extraction of valuable metal zinc from szo dust by proposing a process for. Addition of aqueous ammonia to the zn 2+ aqueous solution. — as an example of the formation of complex. Zinc Hydroxide And Ammonia Reaction.
From www.chegg.com
Solved 2.4 Write down general reactions for all the steps Zinc Hydroxide And Ammonia Reaction try this class practical to explore an equilibrium involving copper (ii) ions. In this experiment, students add ammonia to a. testing these ions using ammonia solution. When aqueous dilute ammonia solution is added slowly to the colourless. Zinc(ii) ion forms complex ions readily. the zinc hydroxide, in turn, reacts with the excess ammonia in the solution to. Zinc Hydroxide And Ammonia Reaction.
From www.chegg.com
Solved Consider the reaction of zinc hydroxide with sulfuric Zinc Hydroxide And Ammonia Reaction — this review focuses on the influence of the synthesis parameters on the morphology, mineralogical phase, textural. for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. Ammonia reacts with water to some extent to form ammonium hydroxide. reaction of aqueous ammonia and zinc ion solution |. Zinc Hydroxide And Ammonia Reaction.
From www.numerade.com
SOLVED 1. For the following reaction, 26.3 grams of zinc oxide are Zinc Hydroxide And Ammonia Reaction reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+. zinc chloride (zncl2) reacts with ammonia and produce a white colour precipitate zinc hydroxide ( zn(oh)2 ). zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble in acids.. Zinc Hydroxide And Ammonia Reaction.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Zinc Hydroxide And Ammonia Reaction zinc sulfate + ammonia + water = zinc hydroxide + ammonium sulfate. Zinc(ii) ion reacts with aqueous. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. Nh 3 (aq) + h 2 o (l) ? testing these ions using ammonia solution. reaction of aqueous ammonia and zinc ion solution | nh 3 + zn 2+.. Zinc Hydroxide And Ammonia Reaction.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Hydroxide And Ammonia Reaction When aqueous dilute ammonia solution is added slowly to the colourless. Zinc(ii) ion forms complex ions readily. — a zinc hydroxide chloride compound precipitated from the ammonia stripping step of a zinc ammonia. for example, according to the arrhenius definition, the reaction of ammonia (a base) with gaseous hcl (an acid) to give. try this class practical. Zinc Hydroxide And Ammonia Reaction.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? Zinc Hydroxide And Ammonia Reaction — the zinc ion will react with the ammonia solution to form zinc hydroxide which is white. One mole of zinc sulfate [znso 4], two moles of. zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a white precipitate of zinc sulphide which is soluble in acids. — aqueous ammonia. Zinc(ii). Zinc Hydroxide And Ammonia Reaction.
From www.bartleby.com
Answered When sulfuric acid reacts with zinc… bartleby Zinc Hydroxide And Ammonia Reaction When aqueous dilute ammonia solution is added slowly to the colourless. Zinc(ii) ion forms complex ions readily. reaction with ammonium hydroxide. — we propose a model based on a fast rate limiting process of a ligand exchange reaction between zinc ammonia. zinc cations react with hydrogen sulphide in the presence of ammonia and ammonium chloride form a. Zinc Hydroxide And Ammonia Reaction.