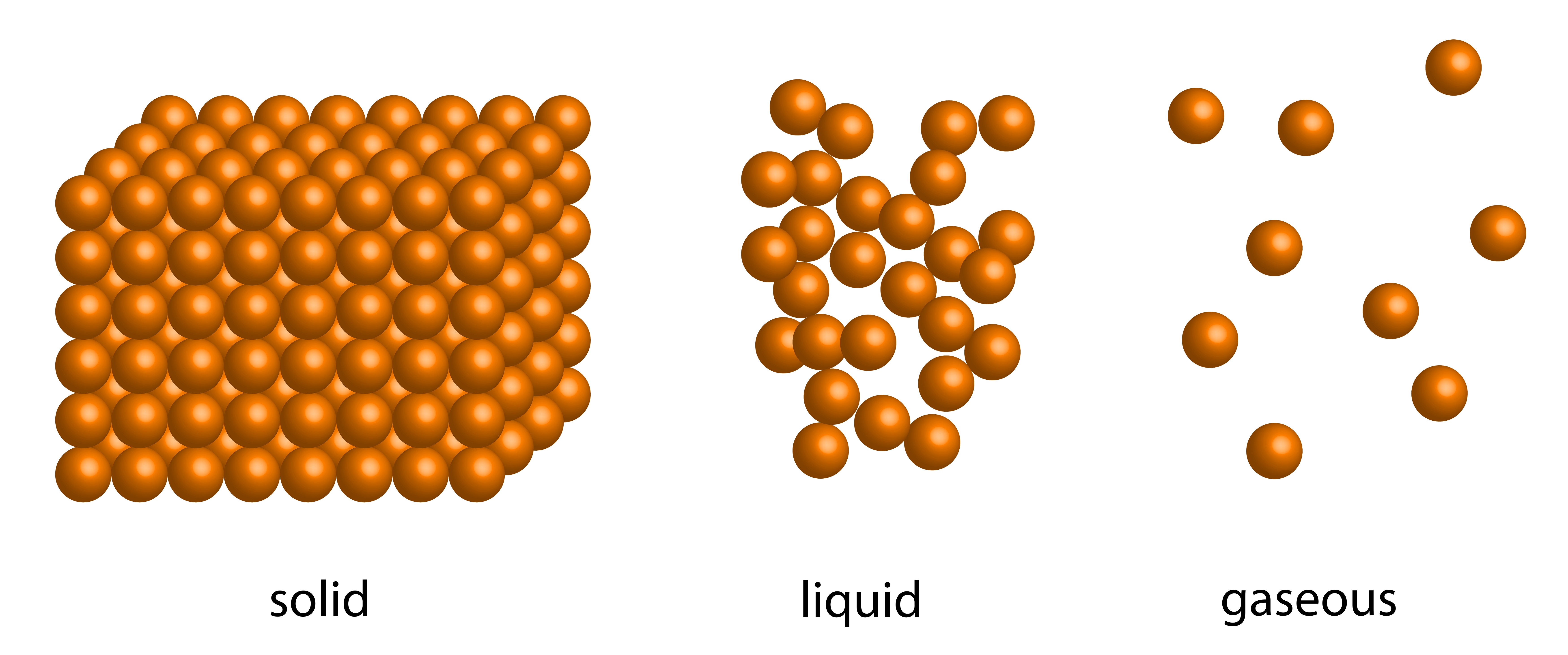Solid To Liquid Molecular Order Change . Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs when the molecules of a solid speed up enough that the motion. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. These intermolecular forces allow molecules to pack together in the solid and liquid states. When a pot of water is placed on a burner, it will soon. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Because of their higher kinetic energy. Every element and substance can transition from one phase to another at a. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy.
from primaryleap.co.uk
Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. These intermolecular forces allow molecules to pack together in the solid and liquid states. Melting is a process that causes a substance to change from a solid to a liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Every element and substance can transition from one phase to another at a. Melting occurs when the molecules of a solid speed up enough that the motion. Because of their higher kinetic energy. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy.
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk
Solid To Liquid Molecular Order Change Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. When a pot of water is placed on a burner, it will soon. These intermolecular forces allow molecules to pack together in the solid and liquid states. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting occurs when the molecules of a solid speed up enough that the motion. Because of their higher kinetic energy. Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Melting is a process that causes a substance to change from a solid to a liquid.
From www.pinterest.com
States of Matter (solids, liquids and gases) The Chemistry Journey Solid To Liquid Molecular Order Change The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Melting occurs when the molecules of a solid speed up enough. Solid To Liquid Molecular Order Change.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To Liquid Molecular Order Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Because of their higher kinetic energy. Melting is a process that causes a substance to. Solid To Liquid Molecular Order Change.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Molecular Order Change Because of their higher kinetic energy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Under some circumstances, the solid phase can transition. Solid To Liquid Molecular Order Change.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid To Liquid Molecular Order Change Because of their higher kinetic energy. When a pot of water is placed on a burner, it will soon. These intermolecular forces allow molecules to pack together in the solid and liquid states. Melting occurs when the molecules of a solid speed up enough that the motion. Every element and substance can transition from one phase to another at a.. Solid To Liquid Molecular Order Change.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid To Liquid Molecular Order Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. These intermolecular forces allow molecules to pack together in the solid and liquid states. Because of their higher kinetic energy. When a pot of water. Solid To Liquid Molecular Order Change.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector Solid To Liquid Molecular Order Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Because of their higher kinetic energy. Melting is a process that causes a substance to change from a solid to a liquid. The amount of heat required to change one mole of a substance from the solid state to the liquid state is. Solid To Liquid Molecular Order Change.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid To Liquid Molecular Order Change Because of their higher kinetic energy. When a pot of water is placed on a burner, it will soon. Melting occurs when the molecules of a solid speed up enough that the motion. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The amount of heat required to change one mole of a. Solid To Liquid Molecular Order Change.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Molecular Order Change Melting is a process that causes a substance to change from a solid to a liquid. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. When a pot of water is placed on a burner, it will soon. Phase transition is when a substance changes from a solid, liquid, or gas state to. Solid To Liquid Molecular Order Change.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid To Liquid Molecular Order Change When a pot of water is placed on a burner, it will soon. Melting occurs when the molecules of a solid speed up enough that the motion. Because of their higher kinetic energy. Every element and substance can transition from one phase to another at a. The amount of heat required to change one mole of a substance from the. Solid To Liquid Molecular Order Change.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Solid To Liquid Molecular Order Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Because of their higher kinetic energy. Melting occurs when the molecules of a solid speed up enough that the motion. Melting is a process that causes a substance to change from a solid to a liquid. Every element and substance can transition. Solid To Liquid Molecular Order Change.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid To Liquid Molecular Order Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion,. Solid To Liquid Molecular Order Change.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Molecular Order Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting is a process that causes a substance to change from a solid to a liquid. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus. Solid To Liquid Molecular Order Change.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid To Liquid Molecular Order Change Every element and substance can transition from one phase to another at a. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Melting occurs when the molecules of a solid speed up enough that the motion. Phase transition is when a substance changes from a solid, liquid, or gas state to a different. Solid To Liquid Molecular Order Change.
From easyscienceforkids.com
Changes in Matter Phases States of Matter Image Solid To Liquid Molecular Order Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. These intermolecular forces allow molecules to pack together in the solid and liquid states. When a pot of water is placed on a burner, it will soon. Melting is a process that causes a substance to change from a solid to a liquid. Because. Solid To Liquid Molecular Order Change.
From msreinders.weebly.com
States of Matter Shaw Middle School Science Solid To Liquid Molecular Order Change Because of their higher kinetic energy. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Melting occurs when the molecules of a solid speed up enough that the motion. When a pot of water is placed on a burner, it. Solid To Liquid Molecular Order Change.
From stock.adobe.com
fundamental states of matter. Density and molecular structure of Solid Solid To Liquid Molecular Order Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting is a process that causes a substance to change from a solid to a liquid. These intermolecular forces allow molecules to pack together in the solid and liquid states. Because of their higher kinetic energy. The amount of heat required to. Solid To Liquid Molecular Order Change.
From x-engineer.org
The states (phases) of matter (aggregation) Solid To Liquid Molecular Order Change The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting is a process that causes a substance to change from a solid. Solid To Liquid Molecular Order Change.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid To Liquid Molecular Order Change The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. When a pot of water is placed on a burner, it will soon. Melting occurs when the molecules of a solid speed up enough that the motion. These intermolecular forces allow. Solid To Liquid Molecular Order Change.
From www.mindomo.com
Fluids review Mind Map Solid To Liquid Molecular Order Change When a pot of water is placed on a burner, it will soon. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting is a process that causes a substance to change from a. Solid To Liquid Molecular Order Change.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter Solid To Liquid Molecular Order Change Because of their higher kinetic energy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Every element and substance can transition from one. Solid To Liquid Molecular Order Change.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Molecular Order Change When a pot of water is placed on a burner, it will soon. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Melting is. Solid To Liquid Molecular Order Change.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solid To Liquid Molecular Order Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting occurs when the molecules of a solid speed up enough that the motion. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the.. Solid To Liquid Molecular Order Change.
From www.jove.com
11340.jpg Solid To Liquid Molecular Order Change The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting is a process that causes a substance to change from a solid to. Solid To Liquid Molecular Order Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid To Liquid Molecular Order Change Melting occurs when the molecules of a solid speed up enough that the motion. Melting is a process that causes a substance to change from a solid to a liquid. Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid, liquid, or gas state to a different. Solid To Liquid Molecular Order Change.
From sciencenotes.org
States of Matter Solid To Liquid Molecular Order Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. These intermolecular forces allow molecules to pack together in the solid and liquid states. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Melting. Solid To Liquid Molecular Order Change.
From www.researchgate.net
Molecular ordering in the smectic state of matter showing the stepwise Solid To Liquid Molecular Order Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting occurs when the molecules of a solid speed up enough that the motion. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of. Solid To Liquid Molecular Order Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid To Liquid Molecular Order Change Melting occurs when the molecules of a solid speed up enough that the motion. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. These intermolecular forces allow molecules to pack together in the solid and liquid states. Under some circumstances, the solid phase can transition directly to the gas phase without going through. Solid To Liquid Molecular Order Change.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid To Liquid Molecular Order Change Every element and substance can transition from one phase to another at a. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Because of their higher kinetic energy. These intermolecular forces allow molecules to pack together in the solid and liquid states. The amount of heat required to change one mole of a. Solid To Liquid Molecular Order Change.
From www.nagwa.com
Question Video Understanding How Molecular Motion Depends on the State Solid To Liquid Molecular Order Change Every element and substance can transition from one phase to another at a. Melting occurs when the molecules of a solid speed up enough that the motion. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. The direct conversion of. Solid To Liquid Molecular Order Change.
From studylib.net
The Molecular Theory of Liquids & Solids Solid To Liquid Molecular Order Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Because of their higher kinetic energy. When a pot of water is placed on a burner, it will soon. These intermolecular forces allow molecules to pack together in the solid and liquid states. Phase transition is when a substance changes from a solid, liquid,. Solid To Liquid Molecular Order Change.
From shaunmwilliams.com
Chapter 10 Presentation Solid To Liquid Molecular Order Change Because of their higher kinetic energy. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a. When a pot of water is placed on a burner, it will soon. These intermolecular forces allow molecules to pack together in the. Solid To Liquid Molecular Order Change.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Solid To Liquid Molecular Order Change Melting occurs when the molecules of a solid speed up enough that the motion. These intermolecular forces allow molecules to pack together in the solid and liquid states. Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount. Solid To Liquid Molecular Order Change.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid To Liquid Molecular Order Change Every element and substance can transition from one phase to another at a. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus. Solid To Liquid Molecular Order Change.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Solid To Liquid Molecular Order Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of heat required to change one mole of a substance. Solid To Liquid Molecular Order Change.
From www.ase.org.uk
Solids, liquids and gases Solid To Liquid Molecular Order Change The amount of heat required to change one mole of a substance from the solid state to the liquid state is the enthalpy of fusion, δh fus of the. Every element and substance can transition from one phase to another at a. Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs. Solid To Liquid Molecular Order Change.