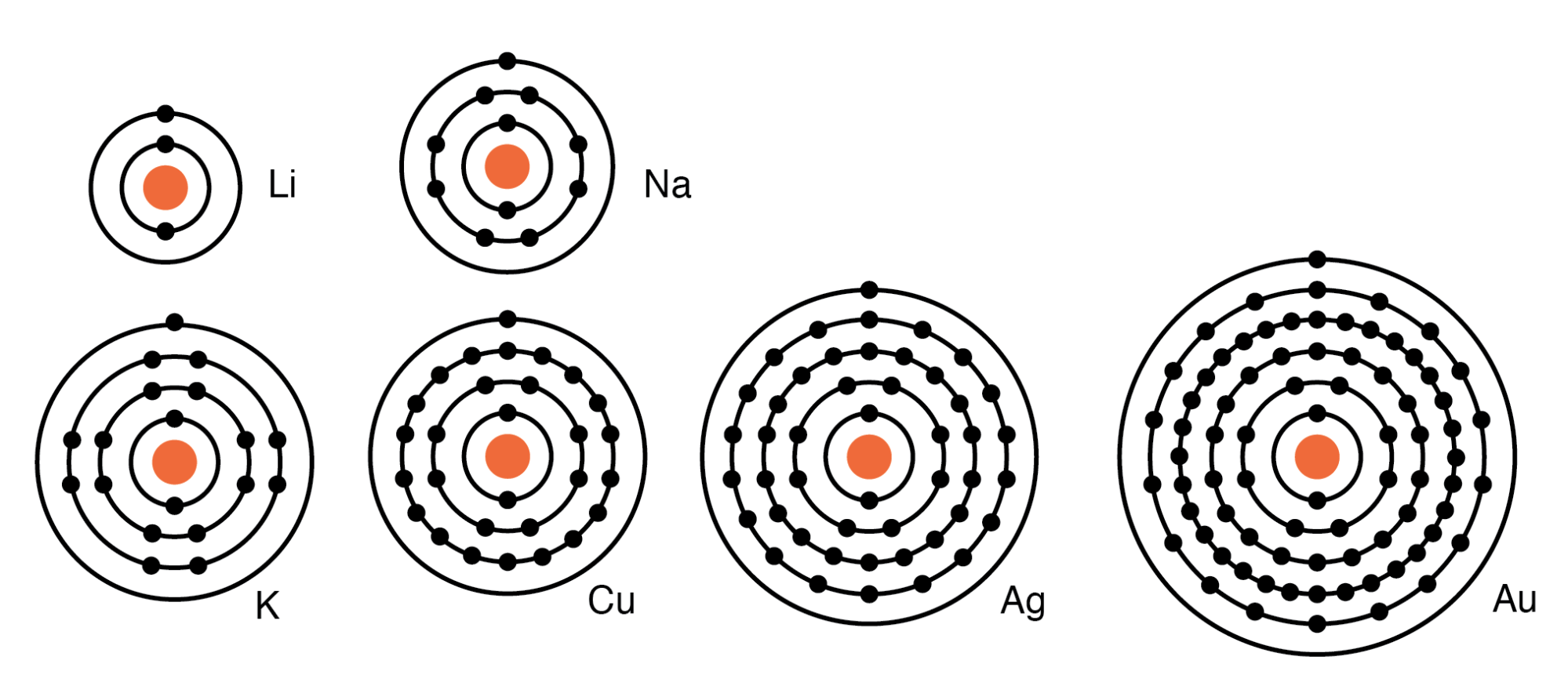
What Is Valence Shell Electron Configuration For I . “electrons in the outer shells that are not filled are called valence electrons”. Identify the block in the periodic table to. Valence electrons are the highest energy electrons in an atom and are. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. In chemistry, valence electrons are the electrons that are located in the outermost. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. A full valence shell is the most. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
from exyrulsiw.blob.core.windows.net
Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. In chemistry, valence electrons are the electrons that are located in the outermost. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Identify the block in the periodic table to. Valence electrons are the highest energy electrons in an atom and are. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). A full valence shell is the most. “electrons in the outer shells that are not filled are called valence electrons”.
What Is The Valence Shell Electron Configuration For All Halogen Atoms
What Is Valence Shell Electron Configuration For I Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. In chemistry, valence electrons are the electrons that are located in the outermost. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. “electrons in the outer shells that are not filled are called valence electrons”. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. A full valence shell is the most. Valence electrons are the highest energy electrons in an atom and are. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Identify the block in the periodic table to.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science What Is Valence Shell Electron Configuration For I “electrons in the outer shells that are not filled are called valence electrons”. Identify the block in the periodic table to. Valence electrons are the highest energy electrons in an atom and are. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Use the periodic table to. What Is Valence Shell Electron Configuration For I.
From byjus.com
Different energy shells, valence shell, valence electrons What Is Valence Shell Electron Configuration For I Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Identify the block in the periodic table to. Valence electrons are the highest energy electrons in an atom and are. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration. What Is Valence Shell Electron Configuration For I.
From www.youtube.com
Valence Electrons and the Periodic Table YouTube What Is Valence Shell Electron Configuration For I A full valence shell is the most. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Find the full electronic configuration and valence. What Is Valence Shell Electron Configuration For I.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. A full valence shell is the most. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Identify. What Is Valence Shell Electron Configuration For I.
From courses.lumenlearning.com
3.4 Electronic Structure of Atoms (Electron Configurations) General What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence is the number of electrons an atom. What Is Valence Shell Electron Configuration For I.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. A full valence shell is the most. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium,. What Is Valence Shell Electron Configuration For I.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. “electrons in the outer shells that are not filled are called valence electrons”. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9.. What Is Valence Shell Electron Configuration For I.
From ceqepxdm.blob.core.windows.net
What Is The Valence Shell Electron Configuration Of Noble Gases at What Is Valence Shell Electron Configuration For I Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. “electrons in the outer shells that are not filled are called valence electrons”. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. In chemistry, valence electrons are the. What Is Valence Shell Electron Configuration For I.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki What Is Valence Shell Electron Configuration For I Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. In chemistry, valence electrons are the electrons that are located in the outermost. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence is the number of electrons an atom. What Is Valence Shell Electron Configuration For I.
From sciencenotes.org
List of Electron Configurations of Elements What Is Valence Shell Electron Configuration For I Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium,. What Is Valence Shell Electron Configuration For I.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is Valence Shell Electron Configuration For I Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium,. What Is Valence Shell Electron Configuration For I.
From selftution.com
Valency and Variable Valency Valence Shell and Electrons » Selftution What Is Valence Shell Electron Configuration For I In chemistry, valence electrons are the electrons that are located in the outermost. “electrons in the outer shells that are not filled are called valence electrons”. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The outermost orbital shell of an atom is called its. What Is Valence Shell Electron Configuration For I.
From klatysxav.blob.core.windows.net
What Is Electron Shell Configuration at Louis Rozier blog What Is Valence Shell Electron Configuration For I “electrons in the outer shells that are not filled are called valence electrons”. A full valence shell is the most. Identify the block in the periodic table to. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence is the number of electrons an atom must lose. What Is Valence Shell Electron Configuration For I.
From cetqppft.blob.core.windows.net
What Is The Characteristic Valence Shell Electron Configuration Of 11Th What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Identify the block in the periodic table to. “electrons in the outer shells that are not filled are called valence electrons”. In chemistry, valence electrons are the electrons that are located. What Is Valence Shell Electron Configuration For I.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Valence Shell Electron Configuration For I Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Valence electrons are the highest energy electrons in an atom and are. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Group 18 elements (helium, neon, and argon. What Is Valence Shell Electron Configuration For I.
From exyrulsiw.blob.core.windows.net
What Is The Valence Shell Electron Configuration For All Halogen Atoms What Is Valence Shell Electron Configuration For I Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. A full valence shell is the most. Valence electrons are the highest energy electrons in an atom and are. In chemistry, valence electrons are the electrons that are located in the outermost. The outermost orbital shell of. What Is Valence Shell Electron Configuration For I.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is Valence Shell Electron Configuration For I Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Use the. What Is Valence Shell Electron Configuration For I.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts What Is Valence Shell Electron Configuration For I A full valence shell is the most. In chemistry, valence electrons are the electrons that are located in the outermost. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. Valence electrons are. What Is Valence Shell Electron Configuration For I.
From biologydictionary.net
Covalent Bond Biology Dictionary What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. We describe an electron configuration. What Is Valence Shell Electron Configuration For I.
From exyrulsiw.blob.core.windows.net
What Is The Valence Shell Electron Configuration For All Halogen Atoms What Is Valence Shell Electron Configuration For I Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Valence electrons are the highest energy electrons in an atom and are. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The outermost orbital shell of an atom is called its valence shell,. What Is Valence Shell Electron Configuration For I.
From cetqppft.blob.core.windows.net
What Is The Characteristic Valence Shell Electron Configuration Of 11Th What Is Valence Shell Electron Configuration For I The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. “electrons in the outer shells that are not filled are called valence electrons”. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. In chemistry, valence electrons are the electrons that. What Is Valence Shell Electron Configuration For I.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is Valence Shell Electron Configuration For I Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Identify the block in the periodic table to. Valence electrons are the highest energy electrons in an atom and are. In chemistry, valence electrons are the electrons that are located in the outermost. Find the full. What Is Valence Shell Electron Configuration For I.
From www.youtube.com
Determine the number of valence electrons from the periodic table and a What Is Valence Shell Electron Configuration For I Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Valence electrons are the highest energy electrons in an atom and are. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The outermost orbital shell of. What Is Valence Shell Electron Configuration For I.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is Valence Shell Electron Configuration For I Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Find the full electronic configuration and valence electrons of any periodic element using. What Is Valence Shell Electron Configuration For I.
From eyy2013.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio What Is Valence Shell Electron Configuration For I Valence electrons are the highest energy electrons in an atom and are. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. “electrons in the outer shells that are not filled are called valence electrons”. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium,. What Is Valence Shell Electron Configuration For I.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Valence Shell Electron Configuration For I Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Valence electrons are the highest energy electrons in an atom and are. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. In chemistry, valence electrons are the electrons. What Is Valence Shell Electron Configuration For I.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is Valence Shell Electron Configuration For I A full valence shell is the most. The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are. “electrons in the outer shells that are not filled are called valence electrons”. Find the full electronic configuration and. What Is Valence Shell Electron Configuration For I.
From mavink.com
Valence Electron Orbital Diagram What Is Valence Shell Electron Configuration For I In chemistry, valence electrons are the electrons that are located in the outermost. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Find the full electronic configuration. What Is Valence Shell Electron Configuration For I.
From fitysand.weebly.com
Periodic table with valence electrons labeled fitysand What Is Valence Shell Electron Configuration For I The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. A full valence shell is the most. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Identify the block in the periodic table to. Valence electrons are the highest energy. What Is Valence Shell Electron Configuration For I.
From graceholloway.z13.web.core.windows.net
Valence Electron Configuration Chart What Is Valence Shell Electron Configuration For I A full valence shell is the most. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. “electrons in the outer shells that are not filled are called valence electrons”. We describe an electron. What Is Valence Shell Electron Configuration For I.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Valence Shell Electron Configuration For I In chemistry, valence electrons are the electrons that are located in the outermost. A full valence shell is the most. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Use the periodic table to predict the valence electron configuration of all the elements of group 2. What Is Valence Shell Electron Configuration For I.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is Valence Shell Electron Configuration For I Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. In chemistry, valence electrons are the electrons that are located in the outermost. Identify the block in the. What Is Valence Shell Electron Configuration For I.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube What Is Valence Shell Electron Configuration For I In chemistry, valence electrons are the electrons that are located in the outermost. Identify the block in the periodic table to. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. “electrons in the outer shells that are not filled are called valence electrons”. Group 18 elements (helium, neon, and argon are. What Is Valence Shell Electron Configuration For I.
From en.m.wikipedia.org
FilePeriodic table of elements showing electron shells.png Wikipedia What Is Valence Shell Electron Configuration For I Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Identify the block in the periodic table to. We describe an electron configuration with a symbol that contains three pieces of information ( figure 15.9.2 15.9. The outermost orbital shell of an atom is called its. What Is Valence Shell Electron Configuration For I.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is Valence Shell Electron Configuration For I In chemistry, valence electrons are the electrons that are located in the outermost. Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. A full valence shell is the most. “electrons in the outer shells that are not filled are called valence electrons”. Identify the block in. What Is Valence Shell Electron Configuration For I.