Two Sources Of Error In A Titration Lab . Titration is a common laboratory technique used to determine the concentration. Titration of a weak acid with a strong base objectives: There are several types of errors that can make titration result differ from the reality. Learn how to calculate and report error and uncertainty in laboratory measurements. To demonstrate the concept of equivalence point in titration. Sources of errors in titration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. This guide covers systematic and random error,. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Errors and precautions in titration experiments introduction.
from www.slideshare.net
Titration is a common laboratory technique used to determine the concentration. There are several types of errors that can make titration result differ from the reality. Errors and precautions in titration experiments introduction. Learn how to calculate and report error and uncertainty in laboratory measurements. To demonstrate the concept of equivalence point in titration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. This guide covers systematic and random error,. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration of a weak acid with a strong base objectives: Sources of errors in titration.
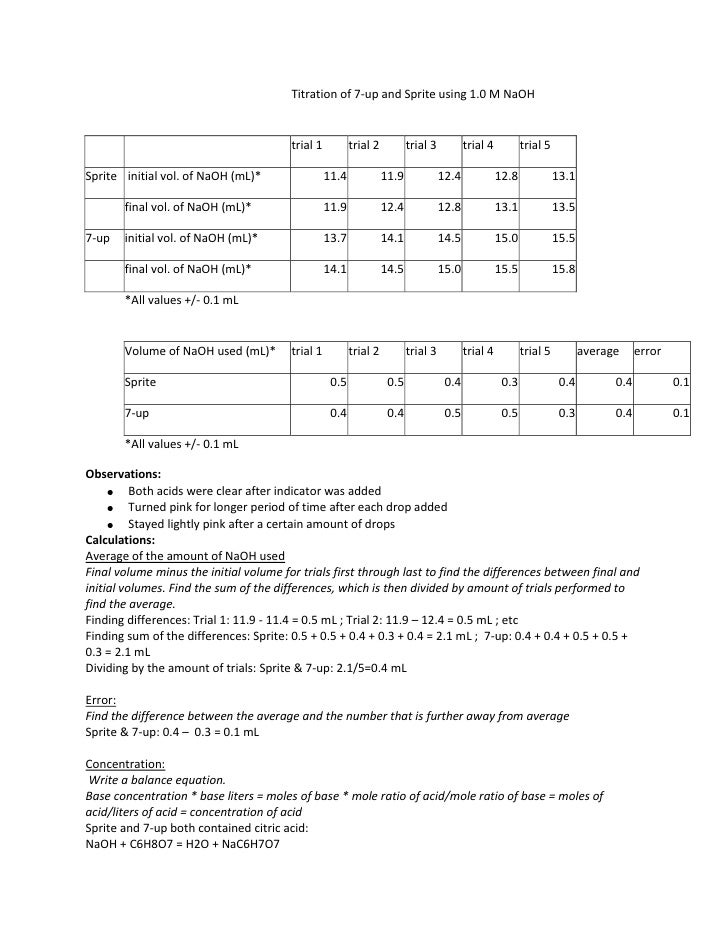
Titration Lab Report
Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in laboratory measurements. To demonstrate the concept of equivalence point in titration. Titration is a common laboratory technique used to determine the concentration. Sources of errors in titration. There are several types of errors that can make titration result differ from the reality. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Errors and precautions in titration experiments introduction. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. This guide covers systematic and random error,. Titration of a weak acid with a strong base objectives:
From www.studocu.com
Error Analysis Example in Titration Chem 3300 Studocu Two Sources Of Error In A Titration Lab There are several types of errors that can make titration result differ from the reality. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Learn how to calculate and report error and uncertainty in laboratory measurements. Titration of a weak acid with a strong base objectives: Titration is. Two Sources Of Error In A Titration Lab.
From fyobkydue.blob.core.windows.net
Titration Lab Percent Error at Anthony Walters blog Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration is a common laboratory technique used to determine the concentration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Sources of errors in titration. There are several types of errors. Two Sources Of Error In A Titration Lab.
From exoohgklo.blob.core.windows.net
Sources Of Error In Chemical Lab at Naomi Freed blog Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration is a common laboratory technique used to determine the concentration. To demonstrate the concept of equivalence point in titration. There are several types of errors that can make titration result differ from the reality. Learn how to calculate. Two Sources Of Error In A Titration Lab.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. Titration of a weak acid with a strong base objectives: Learn how to calculate and report error and uncertainty in laboratory measurements. To demonstrate the concept of equivalence point in titration. Errors and precautions in titration experiments introduction. There are several types of errors that can make titration result. Two Sources Of Error In A Titration Lab.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Two Sources Of Error In A Titration Lab There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in laboratory measurements. Titration of a weak acid with a strong base objectives: To demonstrate the concept of equivalence point in titration. Sources of errors. Two Sources Of Error In A Titration Lab.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry Two Sources Of Error In A Titration Lab There are several types of errors that can make titration result differ from the reality. Learn how to calculate and report error and uncertainty in laboratory measurements. Sources of errors in titration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Errors and precautions in titration experiments introduction. Titration of a weak. Two Sources Of Error In A Titration Lab.
From lawrencegrocabrera.blogspot.com
Sources of Error in Filtration Experiment LawrencegroCabrera Two Sources Of Error In A Titration Lab This guide covers systematic and random error,. To demonstrate the concept of equivalence point in titration. Errors and precautions in titration experiments introduction. There are several types of errors that can make titration result differ from the reality. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. This can cause the recorded. Two Sources Of Error In A Titration Lab.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Two Sources Of Error In A Titration Lab To demonstrate the concept of equivalence point in titration. Titration of a weak acid with a strong base objectives: There are several types of errors that can make titration result differ from the reality. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration is a common laboratory technique used to determine. Two Sources Of Error In A Titration Lab.
From www.youtube.com
Errors in titrations YouTube Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. To demonstrate the concept of equivalence point in titration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration of. Two Sources Of Error In A Titration Lab.
From sciencing.com
Errors in Titration Experiments Sciencing Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To demonstrate the concept of equivalence point in titration. Sources of errors in titration. Errors and precautions in titration experiments introduction. Learn how to calculate and report error and uncertainty in laboratory. Two Sources Of Error In A Titration Lab.
From sciencenotes.org
Systematic vs Random Error Differences and Examples Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration is a common laboratory technique used to determine the concentration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to calculate and report error and uncertainty in laboratory. Two Sources Of Error In A Titration Lab.
From hxeudbkjx.blob.core.windows.net
Titration Lab Examples at Howard Rutherford blog Two Sources Of Error In A Titration Lab Errors and precautions in titration experiments introduction. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration is a common laboratory technique used to determine the concentration. This guide covers systematic and random error,. Learn how to calculate and report error and uncertainty in laboratory measurements. This can cause the recorded volume. Two Sources Of Error In A Titration Lab.
From www.scribd.com
LAB Report 3 Titration Chemistry Two Sources Of Error In A Titration Lab There are several types of errors that can make titration result differ from the reality. This guide covers systematic and random error,. Titration is a common laboratory technique used to determine the concentration. Errors and precautions in titration experiments introduction. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration. Two Sources Of Error In A Titration Lab.
From www.chegg.com
Please write a conclusion for this lab report. It Two Sources Of Error In A Titration Lab Sources of errors in titration. Learn how to calculate and report error and uncertainty in laboratory measurements. This guide covers systematic and random error,. To demonstrate the concept of equivalence point in titration. Titration is a common laboratory technique used to determine the concentration. There are several types of errors that can make titration result differ from the reality. This. Two Sources Of Error In A Titration Lab.
From syatillakmk.blogspot.com
SimplyChemistry Sources of errors in titration Two Sources Of Error In A Titration Lab Sources of errors in titration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To demonstrate the concept of equivalence point in titration. Learn how to calculate and report error and. Two Sources Of Error In A Titration Lab.
From www.slideserve.com
PPT Lecture 2 Bleeding Time PowerPoint Presentation ID308407 Two Sources Of Error In A Titration Lab There are several types of errors that can make titration result differ from the reality. This guide covers systematic and random error,. Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in laboratory measurements. Errors and precautions in titration experiments introduction. Titration of a weak acid with a strong. Two Sources Of Error In A Titration Lab.
From www.vrogue.co
Spot The Errors 1 Youtube vrogue.co Two Sources Of Error In A Titration Lab Titration of a weak acid with a strong base objectives: Titration is a common laboratory technique used to determine the concentration. Errors and precautions in titration experiments introduction. Learn how to calculate and report error and uncertainty in laboratory measurements. Sources of errors in titration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and. Two Sources Of Error In A Titration Lab.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Two Sources Of Error In A Titration Lab Sources of errors in titration. Titration of a weak acid with a strong base objectives: Titration is a common laboratory technique used to determine the concentration. This guide covers systematic and random error,. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To demonstrate the concept of equivalence point in titration. There. Two Sources Of Error In A Titration Lab.
From exosimplo.blob.core.windows.net
What Are The Major Sources Of Error In This Experiment at Raquel Two Sources Of Error In A Titration Lab Errors and precautions in titration experiments introduction. Sources of errors in titration. Learn how to calculate and report error and uncertainty in laboratory measurements. This guide covers systematic and random error,. There are several types of errors that can make titration result differ from the reality. This can cause the recorded volume to be slightly higher or lower than the. Two Sources Of Error In A Titration Lab.
From www.slideserve.com
PPT Measurements and Sources of Errors PowerPoint Presentation, free Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To demonstrate the concept of equivalence point in titration. Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in laboratory measurements. This guide covers systematic and random error,. There are several types. Two Sources Of Error In A Titration Lab.
From studylib.net
AcidBase Titration Answers, Sources of Error Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration of a weak acid with a strong base objectives: This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Learn how to calculate and report error and uncertainty in laboratory measurements.. Two Sources Of Error In A Titration Lab.
From mammothmemory.net
Titration is an experiment to determine concentrations Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Learn how to calculate and report error and uncertainty in laboratory measurements. Errors and precautions in titration experiments introduction. There are several types of errors that can make. Two Sources Of Error In A Titration Lab.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Two Sources Of Error In A Titration Lab Sources of errors in titration. Errors and precautions in titration experiments introduction. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration is a common laboratory technique used to determine the concentration. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent. Two Sources Of Error In A Titration Lab.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Errors and precautions in titration experiments introduction. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. This guide covers systematic and random error,. To demonstrate the concept of equivalence point in. Two Sources Of Error In A Titration Lab.
From www.youtube.com
Analytical Chemistry 1 CH5 L3 Sources of Systematic Error (Slid 4448 Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To demonstrate the concept of equivalence point in titration. This guide covers systematic and random error,. There are several types of errors that can make titration result differ from the reality. This can cause the recorded volume to be slightly higher or lower. Two Sources Of Error In A Titration Lab.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Two Sources Of Error In A Titration Lab This guide covers systematic and random error,. There are several types of errors that can make titration result differ from the reality. To demonstrate the concept of equivalence point in titration. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Errors and precautions in titration experiments introduction. Learn. Two Sources Of Error In A Titration Lab.
From www.youtube.com
Random and Systematic Errors in Titrations YouTube Two Sources Of Error In A Titration Lab Learn how to calculate and report error and uncertainty in laboratory measurements. Errors and precautions in titration experiments introduction. This guide covers systematic and random error,. There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory technique used to determine the concentration. To demonstrate the concept of equivalence point in. Two Sources Of Error In A Titration Lab.
From www.slideshare.net
Titration Lab Report Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory technique used to determine the concentration. Sources of errors in titration. To demonstrate the concept of equivalence point in. Two Sources Of Error In A Titration Lab.
From sciencing.com
Reasons for Error in a Chemistry Experiment Sciencing Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Titration of a weak acid with a strong base objectives: Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in laboratory measurements. Sources of errors in titration. There are several types of. Two Sources Of Error In A Titration Lab.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Two Sources Of Error In A Titration Lab Titration of a weak acid with a strong base objectives: This guide covers systematic and random error,. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Errors and precautions in titration experiments introduction. There are several types of errors that can make titration result differ from the reality.. Two Sources Of Error In A Titration Lab.
From hxevyapvc.blob.core.windows.net
Titration Percentage Error at Adelaide Nix blog Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. There are several types of errors that can make titration result differ from the reality. Titration of a weak acid with a strong base objectives: To demonstrate the concept of equivalence point in titration. Sources of errors in titration.. Two Sources Of Error In A Titration Lab.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration of a weak acid with a strong base objectives: Sources of errors in titration. This guide covers systematic and random error,.. Two Sources Of Error In A Titration Lab.
From hxefauaxo.blob.core.windows.net
Titration Error Analysis at Rosie Gardner blog Two Sources Of Error In A Titration Lab This can cause the recorded volume to be slightly higher or lower than the actual volume, leading to inaccurate titration results. Titration of a weak acid with a strong base objectives: Sources of errors in titration. Learn how to calculate and report error and uncertainty in laboratory measurements. This guide covers systematic and random error,. There are several types of. Two Sources Of Error In A Titration Lab.
From www.slideserve.com
PPT Science, Measurements, Uncertainty and Error PowerPoint Two Sources Of Error In A Titration Lab Titration is a common laboratory technique used to determine the concentration. To demonstrate the concept of equivalence point in titration. Errors and precautions in titration experiments introduction. Learn how to calculate and report error and uncertainty in laboratory measurements. There are several types of errors that can make titration result differ from the reality. Titration of a weak acid with. Two Sources Of Error In A Titration Lab.
From www.slideserve.com
PPT Volumetric Analysis (Titration) PowerPoint Presentation, free Two Sources Of Error In A Titration Lab Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. This guide covers systematic and random error,. There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory technique used to determine the concentration. Learn how to calculate and report error and uncertainty in. Two Sources Of Error In A Titration Lab.