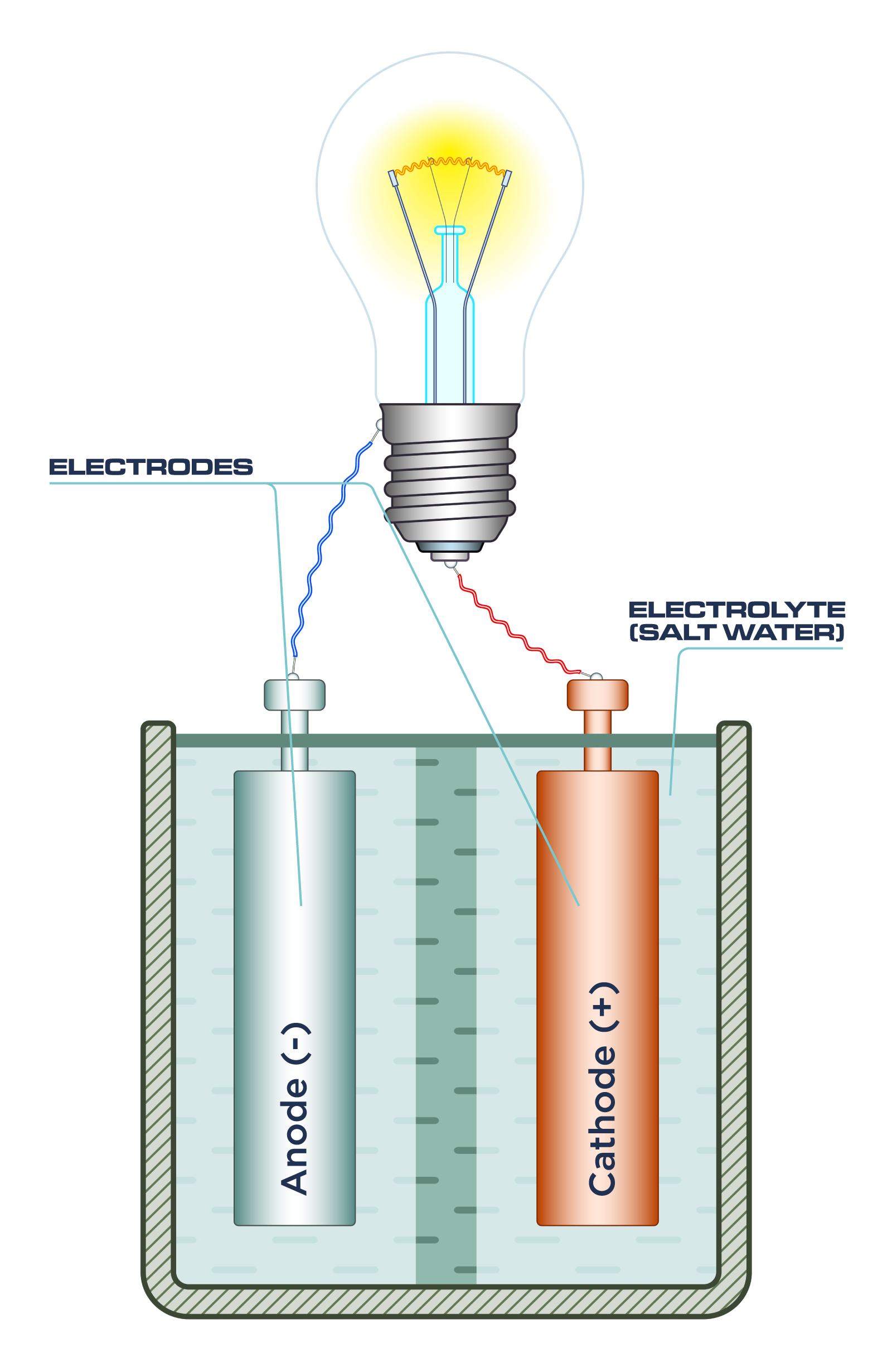
Can Salt Conduct Electricity Without Water . Pure water is not very conductive, and only a tiny bit of current can move through the water. The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. These ions are what carry electricity through the. Ionic compounds like sodium chloride. If the circuit does not. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. When salt or sodium chloride (nacl) is dissolved in it, however, the. The conductivity of the salt water is due to the presence of both positively and negatively. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. No, salt doesn’t conduct electricity. The lattice structure traps the ions, preventing them from moving. Salt includes ions, which function as charged particles, but the ions are not free. Salt in its solid state does not conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity.
from ourfuture.energy
No, salt doesn’t conduct electricity. When salt or sodium chloride (nacl) is dissolved in it, however, the. Salt in its solid state does not conduct electricity. The conductivity of the salt water is due to the presence of both positively and negatively. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. If the circuit does not. Ionic compounds like sodium chloride. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. Salt includes ions, which function as charged particles, but the ions are not free. These ions are what carry electricity through the.
Light from salt OurFuture.Energy
Can Salt Conduct Electricity Without Water If the circuit does not. When salt or sodium chloride (nacl) is dissolved in it, however, the. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. Ionic compounds like sodium chloride. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. These ions are what carry electricity through the. Salt includes ions, which function as charged particles, but the ions are not free. If the circuit does not. Salt in its solid state does not conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. The lattice structure traps the ions, preventing them from moving. The conductivity of the salt water is due to the presence of both positively and negatively. No, salt doesn’t conduct electricity.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Can Salt Conduct Electricity Without Water In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. The conductivity of the salt water is due to the presence of both positively and negatively. Pure water is not very conductive, and only a tiny bit of current can move through the water. When salt or sodium chloride (nacl). Can Salt Conduct Electricity Without Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water Ionic compounds like sodium chloride. Salt in its solid state does not conduct electricity. The lattice structure traps the ions, preventing them from moving. The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt includes ions,. Can Salt Conduct Electricity Without Water.
From dbdalrymplemicrurus.z21.web.core.windows.net
Electricity From Salt Water Can Salt Conduct Electricity Without Water Pure water is not very conductive, and only a tiny bit of current can move through the water. The conductivity of the salt water is due to the presence of both positively and negatively. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. Salt includes ions, which function. Can Salt Conduct Electricity Without Water.
From www.youtube.com
Why Saltwater Conducts Electricity Science Experiment YouTube Can Salt Conduct Electricity Without Water These ions are what carry electricity through the. If the circuit does not. No, salt doesn’t conduct electricity. Ionic compounds like sodium chloride. The lattice structure traps the ions, preventing them from moving. Salt in its solid state does not conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they. Can Salt Conduct Electricity Without Water.
From www.youtube.com
Electrical conductivity with salt water YouTube Can Salt Conduct Electricity Without Water Pure water is not very conductive, and only a tiny bit of current can move through the water. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in. Can Salt Conduct Electricity Without Water.
From www.youtube.com
Why aqueous Acids conduct electricity? Acid , Base and salts Animated lectureJatin academy Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt in its solid state does not conduct electricity. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. The salt (nacl) water solution is an. Can Salt Conduct Electricity Without Water.
From studyzonequaaludes.z13.web.core.windows.net
Salt Water Into Electricity Can Salt Conduct Electricity Without Water In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. These ions are what carry electricity through the. The conductivity of the salt water is due to the presence of both positively and negatively. When salt or sodium chloride (nacl) is dissolved in it, however, the. Salt in its solid. Can Salt Conduct Electricity Without Water.
From www.youtube.com
Electrical Conductivity with salt water & sugar water YouTube Can Salt Conduct Electricity Without Water When salt or sodium chloride (nacl) is dissolved in it, however, the. No, salt doesn’t conduct electricity. The lattice structure traps the ions, preventing them from moving. Salt includes ions, which function as charged particles, but the ions are not free. Pure water is not very conductive, and only a tiny bit of current can move through the water. If. Can Salt Conduct Electricity Without Water.
From www.expii.com
Electrolytes — Definition & Overview Expii Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. Salt includes ions, which function as charged particles, but the ions are not free. Ionic compounds like sodium chloride. The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. These ions are what carry electricity through the. If the circuit does not. A substance needs to have charged particles, like. Can Salt Conduct Electricity Without Water.
From ourfuture.energy
Light from salt OurFuture.Energy Can Salt Conduct Electricity Without Water A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. When salt or sodium chloride (nacl) is dissolved in it, however, the. The salt (nacl) water solution is an. Can Salt Conduct Electricity Without Water.
From www.rookieparenting.com
Electricity Science Projects Can Salt Conduct Electricity Without Water A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. The conductivity of the salt water is due to the presence of both positively and negatively. Salt in its solid state does not conduct electricity. Salt includes ions, which function as charged particles, but the ions are not free.. Can Salt Conduct Electricity Without Water.
From brainly.in
describe an activity to show that solution of salt in water can conduct electricity Brainly.in Can Salt Conduct Electricity Without Water The lattice structure traps the ions, preventing them from moving. Pure water is not very conductive, and only a tiny bit of current can move through the water. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. These ions are what carry electricity through the.. Can Salt Conduct Electricity Without Water.
From www.pinterest.com
Does salt water conduct electricity? How about fresh water? Let's experiment and find out Can Salt Conduct Electricity Without Water When salt or sodium chloride (nacl) is dissolved in it, however, the. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. Salt in its solid state does not conduct electricity. The conductivity of the salt water is due to the presence of both positively and. Can Salt Conduct Electricity Without Water.
From eduforkid.com
What Substance Does Not Conduct Electricity When Dissolved In Water EduForKid Can Salt Conduct Electricity Without Water Salt in its solid state does not conduct electricity. These ions are what carry electricity through the. When salt or sodium chloride (nacl) is dissolved in it, however, the. The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. Salt includes ions, which function as charged particles, but the ions are not free. If the. Can Salt Conduct Electricity Without Water.
From dbdalrymplemicrurus.z21.web.core.windows.net
Electricity From Salt Water Can Salt Conduct Electricity Without Water If the circuit does not. Salt in its solid state does not conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. A substance needs to have charged particles, like. Can Salt Conduct Electricity Without Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. Ionic compounds like sodium chloride. These ions are what carry electricity through the. The conductivity of the salt water is due to the presence of both positively and negatively. When salt or sodium chloride (nacl) is dissolved in it, however, the. The lattice structure traps the ions, preventing them from moving. Pure water is not. Can Salt Conduct Electricity Without Water.
From www.youtube.com
Water Conducts Electricity Experiment Electrical Conductivity with Salt Water Salt Water Can Salt Conduct Electricity Without Water The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. When salt or sodium chloride (nacl) is dissolved in it, however, the. No, salt doesn’t conduct electricity. Ionic compounds like sodium. Can Salt Conduct Electricity Without Water.
From www.slideserve.com
PPT Sugar and Salt Solutions 1 PowerPoint Presentation, free download ID3122227 Can Salt Conduct Electricity Without Water The lattice structure traps the ions, preventing them from moving. Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt includes ions, which function as charged particles, but the ions are not free. Salt in its solid state does not conduct electricity. These ions are what carry electricity through the. In. Can Salt Conduct Electricity Without Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. The conductivity of the salt water is due to the presence of both positively and negatively. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. A. Can Salt Conduct Electricity Without Water.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Can Salt Conduct Electricity Without Water If the circuit does not. Ionic compounds like sodium chloride. No, salt doesn’t conduct electricity. In a saltwater circuit, the brightness of a light bulb is directly proportional to the salt concentration in the water. When salt or sodium chloride (nacl) is dissolved in it, however, the. The lattice structure traps the ions, preventing them from moving. When you put. Can Salt Conduct Electricity Without Water.
From studyzonequaaludes.z13.web.core.windows.net
Salt Water For Electricity Can Salt Conduct Electricity Without Water A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. These ions are what carry electricity through the. The conductivity of the salt water is due to the presence of both positively and negatively. When you put salt in water, the water molecules pull the sodium and chlorine ions. Can Salt Conduct Electricity Without Water.
From klasseddo.blob.core.windows.net
Can Gold Conduct Electricity In Water at Kurt Bruner blog Can Salt Conduct Electricity Without Water If the circuit does not. The lattice structure traps the ions, preventing them from moving. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity.. Can Salt Conduct Electricity Without Water.
From ifunny.co
Pure water does not conduct electricity impurities found in water conduct electricity (such as Can Salt Conduct Electricity Without Water Ionic compounds like sodium chloride. No, salt doesn’t conduct electricity. If the circuit does not. These ions are what carry electricity through the. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. The conductivity of the salt water is due to the presence of both. Can Salt Conduct Electricity Without Water.
From www.youtube.com
How to conduct electricity with salt water? YouTube Can Salt Conduct Electricity Without Water When salt or sodium chloride (nacl) is dissolved in it, however, the. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt in its solid state does not conduct electricity.. Can Salt Conduct Electricity Without Water.
From studylibraryintroit.z14.web.core.windows.net
Electricity From Salt Water Can Salt Conduct Electricity Without Water These ions are what carry electricity through the. Salt includes ions, which function as charged particles, but the ions are not free. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. When salt or sodium chloride (nacl) is dissolved in it, however, the. Ionic compounds like sodium chloride.. Can Salt Conduct Electricity Without Water.
From www.facebook.com
Free Energy Generator Using Salt Water _ Free Energy Science Experiment Free Energy Generator Can Salt Conduct Electricity Without Water The salt (nacl) water solution is an electrolyte solution which is, essentially, a conductive solution. When salt or sodium chloride (nacl) is dissolved in it, however, the. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. Salt in its solid state does not conduct electricity. If the circuit. Can Salt Conduct Electricity Without Water.
From loezuwgau.blob.core.windows.net
How To Accurately Measure The Electrical Conductivity Of A Liquid at Jeremy Chandler blog Can Salt Conduct Electricity Without Water When salt or sodium chloride (nacl) is dissolved in it, however, the. Pure water is not very conductive, and only a tiny bit of current can move through the water. Ionic compounds like sodium chloride. No, salt doesn’t conduct electricity. The conductivity of the salt water is due to the presence of both positively and negatively. The lattice structure traps. Can Salt Conduct Electricity Without Water.
From electricity.selotips.com
How Do Ions Conduct Electricity Can Salt Conduct Electricity Without Water These ions are what carry electricity through the. When salt or sodium chloride (nacl) is dissolved in it, however, the. Ionic compounds like sodium chloride. If the circuit does not. The lattice structure traps the ions, preventing them from moving. No, salt doesn’t conduct electricity. A substance needs to have charged particles, like ions and electrons that are free to. Can Salt Conduct Electricity Without Water.
From lessonlistblastoderm.z4.web.core.windows.net
Why Does Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. Pure water is not very conductive, and only a tiny bit of current can move through the water. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. Salt includes ions, which function as charged particles, but the ions are not free. The conductivity. Can Salt Conduct Electricity Without Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt includes ions, which function as charged particles, but the ions are not. Can Salt Conduct Electricity Without Water.
From www.youtube.com
electrical conductivity with salt water working model school science exhibition project YouTube Can Salt Conduct Electricity Without Water Pure water is not very conductive, and only a tiny bit of current can move through the water. No, salt doesn’t conduct electricity. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. When salt or sodium chloride (nacl) is dissolved in it, however, the. A. Can Salt Conduct Electricity Without Water.
From howtofunda.com
electrical conductivity experiment using saltwater Free Science Maths English Physics Can Salt Conduct Electricity Without Water The conductivity of the salt water is due to the presence of both positively and negatively. When salt or sodium chloride (nacl) is dissolved in it, however, the. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. If the circuit does not. Pure water is not very conductive,. Can Salt Conduct Electricity Without Water.
From lessonschoolworkday.z14.web.core.windows.net
Will Salt Water Conduct Electricity Can Salt Conduct Electricity Without Water No, salt doesn’t conduct electricity. These ions are what carry electricity through the. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. The conductivity of the salt water is due to the presence of both positively and negatively. When salt or sodium chloride (nacl) is dissolved in it,. Can Salt Conduct Electricity Without Water.
From www.slideserve.com
PPT ELECTROLYSIS A guide for GCSE students PowerPoint Presentation, free download ID297164 Can Salt Conduct Electricity Without Water Pure water is not very conductive, and only a tiny bit of current can move through the water. Salt in its solid state does not conduct electricity. The lattice structure traps the ions, preventing them from moving. When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the. Can Salt Conduct Electricity Without Water.
From www.chegg.com
Solved Question 23 A salt that can conduct electricity when Can Salt Conduct Electricity Without Water Salt in its solid state does not conduct electricity. These ions are what carry electricity through the. A substance needs to have charged particles, like ions and electrons that are free to travel through it to conduct electricity. When salt or sodium chloride (nacl) is dissolved in it, however, the. Pure water is not very conductive, and only a tiny. Can Salt Conduct Electricity Without Water.