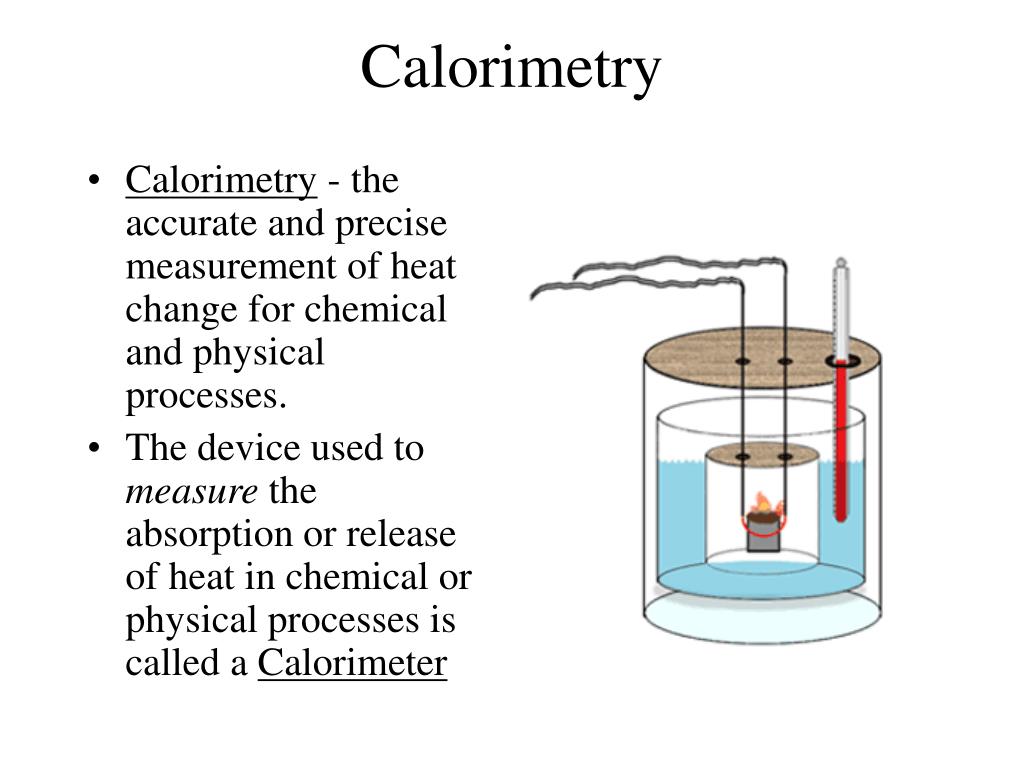
What Is Calorimetry The Study Of . To do so, the heat is exchanged with a calibrated object. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Chemists use calorimetry to determine the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It uses devices called calorimeters , which measure the change in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
from www.slideserve.com
To do so, the heat is exchanged with a calibrated object. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. It uses devices called calorimeters , which measure the change in. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry.
PPT Chapter 11 PowerPoint Presentation, free download ID1084959
What Is Calorimetry The Study Of Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry to determine the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. It uses devices called calorimeters , which measure the change in. To do so, the heat is exchanged with a calibrated object. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
From studylib.net
PPT Calorimetry What Is Calorimetry The Study Of It uses devices called calorimeters , which measure the change in. To do so, the heat is exchanged with a calibrated object. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The act. What Is Calorimetry The Study Of.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of What Is Calorimetry The Study Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the. What Is Calorimetry The Study Of.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog What Is Calorimetry The Study Of The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. What Is Calorimetry The Study Of.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is Calorimetry The Study Of The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. What Is Calorimetry The Study Of.
From sites.google.com
Calorimetry Preliminary HSC Chemistry What Is Calorimetry The Study Of Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is used to measure amounts of heat transferred to or from a substance. The act or. What Is Calorimetry The Study Of.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube What Is Calorimetry The Study Of Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry. What Is Calorimetry The Study Of.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is Calorimetry The Study Of To do so, the heat is exchanged with a calibrated object. It uses devices called calorimeters , which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Chemists use calorimetry. What Is Calorimetry The Study Of.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Is Calorimetry The Study Of Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is exchanged with a calibrated object. The act or science of measuring the changes in the state variables of a body in order to. What Is Calorimetry The Study Of.
From studylib.net
Calorimetry What Is Calorimetry The Study Of In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. What Is Calorimetry The Study Of.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Docsity What Is Calorimetry The Study Of In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is. What Is Calorimetry The Study Of.
From www.chemistryspace.com
ThermoChemistry Calorimetry What Is Calorimetry The Study Of Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. It uses devices called calorimeters , which measure the change in. To do so, the heat is exchanged with a calibrated object. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. What Is Calorimetry The Study Of.
From studyfinder.org
How to Use a Calorimetry Gizmo to Get the Answers You Need What Is Calorimetry The Study Of In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. It uses devices called calorimeters , which measure the change in. Chemists use calorimetry to determine the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with. What Is Calorimetry The Study Of.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimetry The Study Of Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. What Is Calorimetry The Study Of.
From rachel-study-exams.blogspot.com
Calorimetry What Is The Relationship Answers 44+ Pages Summary in Doc [2.8mb] Updated Rachel What Is Calorimetry The Study Of Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the measurement. What Is Calorimetry The Study Of.
From www.youtube.com
050 Calorimetry YouTube What Is Calorimetry The Study Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure. What Is Calorimetry The Study Of.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Is Calorimetry The Study Of The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. It uses devices called calorimeters , which measure the change in. Calorimetry is used to measure amounts of. What Is Calorimetry The Study Of.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimetry The Study Of Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry. What Is Calorimetry The Study Of.
From dvisavzveco.blob.core.windows.net
What Is Calorimetry Thermal Physics at Mary Murphy blog What Is Calorimetry The Study Of Chemists use calorimetry to determine the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such. What Is Calorimetry The Study Of.
From dvisavzveco.blob.core.windows.net
What Is Calorimetry Thermal Physics at Mary Murphy blog What Is Calorimetry The Study Of To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Chemists use calorimetry to determine the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the. What Is Calorimetry The Study Of.
From 2012books.lardbucket.org
Calorimetry What Is Calorimetry The Study Of Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry to determine the. To do so, the heat is exchanged with a calibrated object. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is. What Is Calorimetry The Study Of.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimetry The Study Of To do so, the heat is exchanged with a calibrated object. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters , which measure the change in. Calorimetry is the measurement of. What Is Calorimetry The Study Of.
From studylib.net
experiment 9 Thermochemistry Calorimetry What Is Calorimetry The Study Of Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Chemists use calorimetry to determine the. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. What Is Calorimetry The Study Of.
From www.studypool.com
SOLUTION Study material on calorimetry Studypool What Is Calorimetry The Study Of It uses devices called calorimeters , which measure the change in. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The act or science of measuring the changes in the state variables of a. What Is Calorimetry The Study Of.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is Calorimetry The Study Of Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with. What Is Calorimetry The Study Of.
From studylib.net
Calorimetry What Is Calorimetry The Study Of Chemists use calorimetry to determine the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during. What Is Calorimetry The Study Of.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimetry The Study Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the set of techniques used to measure enthalpy. What Is Calorimetry The Study Of.
From www.slideserve.com
PPT Chapter 16 Calorimetry PowerPoint Presentation, free download ID3593495 What Is Calorimetry The Study Of In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object. It uses devices called calorimeters , which measure the change in. Calorimetry is used. What Is Calorimetry The Study Of.
From studylib.net
The Principles of Calorimetry What Is Calorimetry The Study Of Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine. What Is Calorimetry The Study Of.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Calorimetry The Study Of To do so, the heat is exchanged with a calibrated object. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. The act or science of measuring the changes in the state. What Is Calorimetry The Study Of.
From byjus.com
State the Principle of calorimetry What Is Calorimetry The Study Of Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine the. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to. What Is Calorimetry The Study Of.
From www.indirectcalorimetry.net
Understanding Indirect Calorimetry Indirect Calorimetry What Is Calorimetry The Study Of The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. To do so, the heat is exchanged with a calibrated object. Calorimetry is the process of measuring the. What Is Calorimetry The Study Of.
From slideplayer.com
Drill How should we set up a lab notebook? What should we include, etc? ppt download What Is Calorimetry The Study Of Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Chemists use calorimetry to determine the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is. What Is Calorimetry The Study Of.
From slideplayer.com
Calorimetry Chapter ppt download What Is Calorimetry The Study Of The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. What Is Calorimetry The Study Of.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 What Is Calorimetry The Study Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act. What Is Calorimetry The Study Of.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch What Is Calorimetry The Study Of To do so, the heat is exchanged with a calibrated object. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry is the set of techniques used to measure. What Is Calorimetry The Study Of.