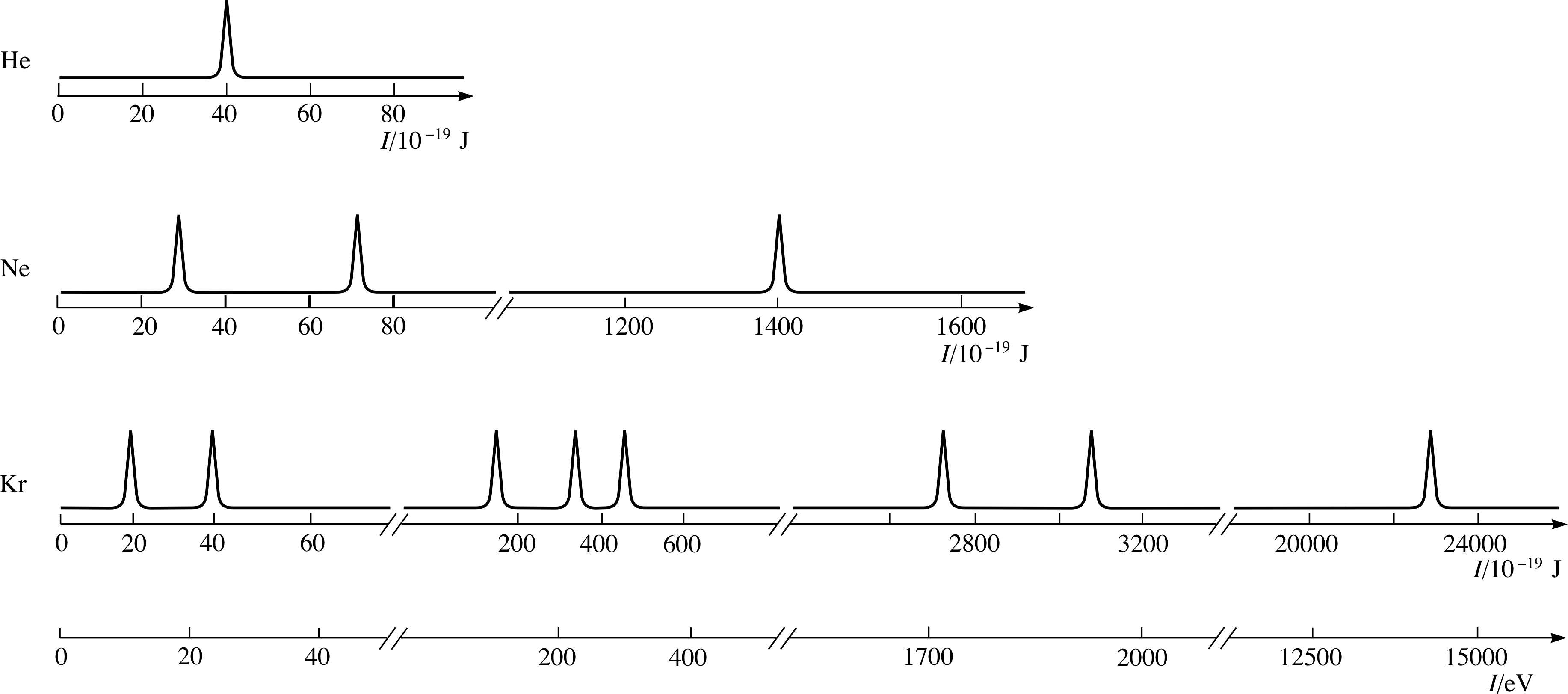
Worksheet Photoelectron Spectroscopy & Electron Configuration . Refer to the pes spectrum below. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. That is, we considered data for an atom’s. Label each peak with the appropriate shell and subshell. In pes, an atom is. Hotoelectron spectroscopy & electron configuration 1. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Write the full electron configuration of sulfur. Refer to the pes spectrum below. How many electrons are represented in each peak? Consider the following pes spectrum a. Make note of the relative. The data we have examined thus far is limited to a single valence electron; Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. How many electrons does the atom contain?
from chessmuseum.org
How many electrons are represented in each peak? Using the plot, write the electron configuration of the element, and identify it. Make note of the relative. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Label each peak with the appropriate shell and subshell. In pes, an atom is. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. The data we have examined thus far is limited to a single valence electron; Hotoelectron spectroscopy & electron configuration 1. That is, we considered data for an atom’s.
50 Photoelectron Spectroscopy Worksheet Answers
Worksheet Photoelectron Spectroscopy & Electron Configuration Using the plot, write the electron configuration of the element, and identify it. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Consider the following pes spectrum a. How many electrons does the atom contain? Label each peak with the appropriate shell and subshell. In pes, an atom is. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. The data we have examined thus far is limited to a single valence electron; Refer to the pes spectrum below. Refer to the pes spectrum below. That is, we considered data for an atom’s. Hotoelectron spectroscopy & electron configuration 1. Using the plot, write the electron configuration of the element, and identify it. Write the full electron configuration of sulfur. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Make note of the relative.
From db-excel.com
Ap Chemistry Photoelectron Spectroscopy Worksheet — Worksheet Photoelectron Spectroscopy & Electron Configuration Consider the following pes spectrum a. Hotoelectron spectroscopy & electron configuration 1. How many electrons are represented in each peak? In pes, an atom is. That is, we considered data for an atom’s. How many electrons does the atom contain? Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.studocu.com
Photoelectron Spectroscopy WorksheetKEY Photoelectron Spectroscopy (PES) ANSWER KEY 1. In a Worksheet Photoelectron Spectroscopy & Electron Configuration In pes, an atom is. Consider the following pes spectrum a. Refer to the pes spectrum below. Using the plot, write the electron configuration of the element, and identify it. How many electrons are represented in each peak? Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. Write the full electron. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From davida.davivienda.com
Worksheet Photoelectron Spectroscopy & Electron Configuration Printable Word Searches Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. How many electrons are represented in each peak? The data we have examined thus far is limited to a single valence electron; Make note of the relative. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From classschooltrommler.z19.web.core.windows.net
Photoelectron Spectroscopy Pes Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Refer to the pes spectrum below. Make note of the relative. The data we have examined thus far is limited to a single valence electron; Photoelectron spectroscopy (pes) is a technique that is used. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From db-excel.com
Ap Chemistry Photoelectron Spectroscopy Worksheet — Worksheet Photoelectron Spectroscopy & Electron Configuration Make note of the relative. Write the full electron configuration of sulfur. Refer to the pes spectrum below. How many electrons are represented in each peak? How many electrons does the atom contain? Consider the following pes spectrum a. Hotoelectron spectroscopy & electron configuration 1. In pes, an atom is. Refer to the pes spectrum below. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.e-streetlight.com
Electron Configuration Practice Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration Hotoelectron spectroscopy & electron configuration 1. Refer to the pes spectrum below. Refer to the pes spectrum below. How many electrons are represented in each peak? How many electrons does the atom contain? Label each peak with the appropriate shell and subshell. Write the full electron configuration of sulfur. Label each peak in the spectrum to show which subshell it. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.onlineworksheet.my.id
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Make note of the relative. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Photoelectron spectroscopy (pes) is a technique that is used to. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From studylib.net
1 Photoelectron Spectroscopy Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration How many electrons does the atom contain? Hotoelectron spectroscopy & electron configuration 1. Make note of the relative. Refer to the pes spectrum below. How many electrons are represented in each peak? That is, we considered data for an atom’s. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. Given the. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From martindxmguide.blogspot.com
32 Photoelectron Spectroscopy Worksheet Answers support worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration That is, we considered data for an atom’s. Using the plot, write the electron configuration of the element, and identify it. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Label each peak with the appropriate shell and subshell. How many electrons does. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From liveworksheetbybeverley.netlify.app
Photoelectron Spectroscopy Ap Chemistry Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Using the plot, write the electron configuration of the element, and identify it. Write the full electron configuration of sulfur. Hotoelectron spectroscopy & electron configuration 1. Make note of the relative. Label each peak. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.pinterest.com
Photoelectron Spectroscopy Worksheet Answers Electron configuration, Worksheet template Worksheet Photoelectron Spectroscopy & Electron Configuration Hotoelectron spectroscopy & electron configuration 1. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Refer to the pes spectrum below. In pes, an atom is. Write the full electron configuration of sulfur. Make note of the relative. Consider the following pes spectrum a. Label each peak with the appropriate. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From db-excel.com
Ap Chemistry Photoelectron Spectroscopy Worksheet — Worksheet Photoelectron Spectroscopy & Electron Configuration How many electrons does the atom contain? Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Label each peak with the appropriate shell and subshell. Refer to the pes spectrum below. Refer to the pes spectrum below. Using the plot, write the electron configuration of the element, and identify it.. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.pinterest.fr
the text is shown in black and white, with an image of numbers on it Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Refer to the pes spectrum below. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. Make note of the relative. Hotoelectron spectroscopy & electron configuration. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.pinterest.com
Recently updated! Photoelectron Spectroscopy & Electron Configuration Lesson, Guided Notes and Worksheet Photoelectron Spectroscopy & Electron Configuration Make note of the relative. That is, we considered data for an atom’s. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. How many electrons does the atom contain? Label each peak in the spectrum to show which subshell it represents (i.e., 1s,. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.artofit.org
Photoelectron spectroscopy worksheet answers worksheet for education Artofit Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Label each peak with the appropriate shell and subshell. Hotoelectron spectroscopy & electron configuration 1. The data we have examined thus far is limited to a single valence electron; Make note of the relative. How many electrons does the atom contain?. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From chessmuseum.org
50 Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Consider the following pes spectrum a. Label each peak with the appropriate shell and subshell. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Write the full electron configuration of sulfur. In pes, an atom is. The data we have examined thus far. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From wordworksheet.com
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak with the appropriate shell and subshell. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Consider the following pes spectrum a. Write the full electron configuration of sulfur. How many electrons are represented in each peak? Hotoelectron spectroscopy & electron configuration 1. Refer to the pes spectrum. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.onlineworksheet.my.id
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Make note of the relative. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. How many electrons are represented in each peak? Refer to the pes spectrum below. In pes, an atom is. That is, we considered data for an atom’s. Photoelectron spectroscopy (pes) is a technique that is used. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From db-excel.com
Photoelectron Spectroscopy Worksheet Answers — Worksheet Photoelectron Spectroscopy & Electron Configuration That is, we considered data for an atom’s. How many electrons are represented in each peak? Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. Refer to the pes spectrum below. Label each peak with the appropriate shell and subshell. Make note of the relative. Refer to the pes spectrum below.. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.onlineworksheet.my.id
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Hotoelectron spectroscopy & electron configuration 1. Refer to the pes spectrum below. In pes, an atom is. Label each peak with the appropriate shell and subshell. Refer to the pes spectrum below. That is,. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From worksheetlibmarko.z19.web.core.windows.net
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Refer to the pes spectrum below. Make note of the relative. Consider the following pes spectrum a. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. The data we have examined thus far is limited to a single valence electron; How many electrons does the atom contain? Write the full electron. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.englishworksheet.my.id
Photoelectron Spectroscopy Worksheet Answers Englishworksheet.my.id Worksheet Photoelectron Spectroscopy & Electron Configuration Refer to the pes spectrum below. Write the full electron configuration of sulfur. Make note of the relative. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Hotoelectron spectroscopy & electron configuration 1. That is, we considered data for an atom’s. The data. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From davida.davivienda.com
Worksheet Photoelectron Spectroscopy & Electron Configuration Printable Word Searches Worksheet Photoelectron Spectroscopy & Electron Configuration How many electrons does the atom contain? Label each peak with the appropriate shell and subshell. Refer to the pes spectrum below. Consider the following pes spectrum a. Hotoelectron spectroscopy & electron configuration 1. Write the full electron configuration of sulfur. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.pinterest.com
Photoelectron Spectroscopy Worksheet Answers Inspirational Ap Chemistry Big Idea 1 Electron Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Write the full electron configuration of sulfur. How many electrons does the atom contain? In pes, an atom is. That is, we considered data for an atom’s. Hotoelectron spectroscopy & electron configuration 1. Consider. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.onlineworksheet.my.id
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Refer to the pes spectrum below. Make note of the relative. Hotoelectron spectroscopy & electron configuration 1. In pes, an atom is. Refer to the pes spectrum below. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From materiallibrarybevan77.z19.web.core.windows.net
Ap Chem Photoelectron Spectroscopy Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration How many electrons are represented in each peak? Refer to the pes spectrum below. Hotoelectron spectroscopy & electron configuration 1. Consider the following pes spectrum a. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. In pes, an atom is. The data we have examined thus far is limited to a. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From studylib.net
Photoelectron Spectroscopy Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak with the appropriate shell and subshell. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Consider the following pes spectrum a. That is, we considered data for an atom’s. The data we have examined thus far is limited to a single valence electron; Make note of the. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From db-excel.com
Ap Chemistry Photoelectron Spectroscopy Worksheet — Worksheet Photoelectron Spectroscopy & Electron Configuration How many electrons are represented in each peak? The data we have examined thus far is limited to a single valence electron; Refer to the pes spectrum below. Write the full electron configuration of sulfur. Using the plot, write the electron configuration of the element, and identify it. That is, we considered data for an atom’s. Given the photoelectron spectra. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.scribd.com
Photoelectron Spectroscopy WorksheetKEY PDF Electron Configuration Electron Worksheet Photoelectron Spectroscopy & Electron Configuration That is, we considered data for an atom’s. Make note of the relative. Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. Consider the following pes spectrum a. Refer to the pes spectrum below. Write the full electron configuration of sulfur. Label each. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From liveworksheetbybeverley.netlify.app
Photoelectron Spectroscopy Ap Chemistry Worksheet Worksheet Photoelectron Spectroscopy & Electron Configuration Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Using the plot, write the electron configuration of the element, and identify it. Hotoelectron spectroscopy & electron configuration 1. Label each peak with the appropriate shell and subshell. The data we have examined thus far is limited to a single valence. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.scribd.com
5 Topic 2 Worksheet 5 Photoelectron Spectroscopy ST PDF Electron Configuration Molecular Worksheet Photoelectron Spectroscopy & Electron Configuration The data we have examined thus far is limited to a single valence electron; Refer to the pes spectrum below. Consider the following pes spectrum a. Make note of the relative. Refer to the pes spectrum below. In pes, an atom is. Hotoelectron spectroscopy & electron configuration 1. How many electrons are represented in each peak? That is, we considered. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.chemistrylearner.com
Free Printable Electron Configuration Worksheets Worksheet Photoelectron Spectroscopy & Electron Configuration Given the photoelectron spectra above for phosphorus, p, and sulfur, s, which of the following best explains why the 2p peak for s is further to. The data we have examined thus far is limited to a single valence electron; How many electrons does the atom contain? Using the plot, write the electron configuration of the element, and identify it.. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From db-excel.com
Ap Chemistry Photoelectron Spectroscopy Worksheet — Worksheet Photoelectron Spectroscopy & Electron Configuration Refer to the pes spectrum below. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. How many electrons are represented in each peak? Label each peak with the appropriate shell and subshell.. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From www.chemistrylearner.com
Free Printable Electron Configuration Worksheets Worksheet Photoelectron Spectroscopy & Electron Configuration Using the plot, write the electron configuration of the element, and identify it. Make note of the relative. Hotoelectron spectroscopy & electron configuration 1. Photoelectron spectroscopy (pes) is a technique that is used to gather information about the electrons in an atom. That is, we considered data for an atom’s. Consider the following pes spectrum a. Label each peak with. Worksheet Photoelectron Spectroscopy & Electron Configuration.
From wordworksheet.com
Photoelectron Spectroscopy Worksheet Answers Worksheet Photoelectron Spectroscopy & Electron Configuration Refer to the pes spectrum below. Label each peak with the appropriate shell and subshell. Refer to the pes spectrum below. Consider the following pes spectrum a. Using the plot, write the electron configuration of the element, and identify it. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. How. Worksheet Photoelectron Spectroscopy & Electron Configuration.