Titration Curve H2Co3 . 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Our goal is to sketch the titration curve quickly,. The figure below shows two titration curves: The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Hcl) or to a weak acid (e.g. Naoh) added either to a strong acid (e.g. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Figure 2 the titration curves of a strong base. The volume of the titrant added. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity.
from www.chegg.com
The volume of the titrant added. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The figure below shows two titration curves: Our goal is to sketch the titration curve quickly,. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Hcl) or to a weak acid (e.g. Naoh) added either to a strong acid (e.g. Figure 2 the titration curves of a strong base. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity.
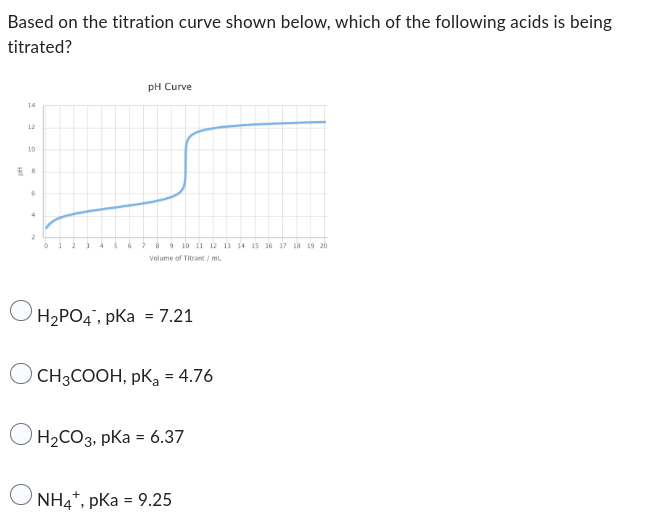
Solved Based on the titration curve shown below, which of
Titration Curve H2Co3 The figure below shows two titration curves: The volume of the titrant added. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Naoh) added either to a strong acid (e.g. Hcl) or to a weak acid (e.g. Our goal is to sketch the titration curve quickly,. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Figure 2 the titration curves of a strong base. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The figure below shows two titration curves:
From www.youtube.com
and Analytical Chemistry Lab. Titration of NaOH and Na2CO3 Titration Curve H2Co3 The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. The figure below shows two titration curves: Figure 2 the titration curves of. Titration Curve H2Co3.
From www.slideserve.com
PPT Fractions of H 2 CO 3 , HCO 3 , and CO 3 2 as a Function of pH Titration Curve H2Co3 The volume of the titrant added. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Hcl) or to a weak acid (e.g. Figure 2 the titration curves of a strong base. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant. Titration Curve H2Co3.
From www.numerade.com
SOLVED 8 Shown bclow is titration curve for carbonic acid verzus Titration Curve H2Co3 The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. Figure 2 the titration curves of a strong base. The course of a titration can be followed. Titration Curve H2Co3.
From www.coursehero.com
[Solved] Use the program to prepare the titration curves for the Titration Curve H2Co3 Our goal is to sketch the titration curve quickly,. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Hcl) or to a weak acid (e.g. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for. Titration Curve H2Co3.
From www.researchgate.net
Typical acid titration curve for hydroxide carbonate system. Download Titration Curve H2Co3 The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The figure below shows two titration curves: Our goal is to sketch the titration curve quickly,. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. Figure. Titration Curve H2Co3.
From www.chegg.com
Solved Based on the titration curve shown below, which of Titration Curve H2Co3 Hcl) or to a weak acid (e.g. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Figure 2 the titration curves of a strong base. The. Titration Curve H2Co3.
From derangedphysiology.com
Buffering in acute respiratory acidbase disturbances Deranged Physiology Titration Curve H2Co3 Naoh) added either to a strong acid (e.g. Our goal is to sketch the titration curve quickly,. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about. Titration Curve H2Co3.
From mungfali.com
Titration Curve HCl And NaOH Titration Curve H2Co3 Figure 2 the titration curves of a strong base. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Hcl) or to a weak acid (e.g. The volume of the titrant added. The shape of a titration curve, a plot of ph versus the amount of acid or base. Titration Curve H2Co3.
From chart-studio.plotly.com
H2SO3 Titration Curve scatter chart made by Harrisonw19 plotly Titration Curve H2Co3 Figure 2 the titration curves of a strong base. Naoh) added either to a strong acid (e.g. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about. Titration Curve H2Co3.
From www.coursehero.com
[Solved] ii. Below is a titration curve for carbonic acid. Indicate key Titration Curve H2Co3 Figure 2 the titration curves of a strong base. The volume of the titrant added. Our goal is to sketch the titration curve quickly,. Hcl) or to a weak acid (e.g. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The figure below shows two titration curves: 1) for $\pu{10. Titration Curve H2Co3.
From slideplayer.com
Dr. Fred Omega Garces Chemistry 201 Miramar College ppt download Titration Curve H2Co3 The figure below shows two titration curves: 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. Our goal is to sketch the titration curve quickly,. Figure 2 the titration. Titration Curve H2Co3.
From www.researchgate.net
(a) Acidbase titration curve of ink and (b) the calculated protonation Titration Curve H2Co3 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Naoh) added either to a strong acid (e.g. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Our goal is to sketch the titration curve. Titration Curve H2Co3.
From www.chegg.com
Solved The following titration curve shows the titration of Titration Curve H2Co3 Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Figure 2 the titration curves of a strong base. The figure below shows two titration curves: The shape of a. Titration Curve H2Co3.
From mavink.com
Titration Labeled Titration Curve H2Co3 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The course of a titration can be followed by plotting the ph of the solution as a function of the. Titration Curve H2Co3.
From www.chegg.com
Solved The curve shows the titration of H2SO3 with NaOH. The Titration Curve H2Co3 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The volume of the titrant added. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The course of a titration can be followed by. Titration Curve H2Co3.
From www.numerade.com
SOLVED What is the area of the titration curve for carbonic acid Titration Curve H2Co3 Our goal is to sketch the titration curve quickly,. Naoh) added either to a strong acid (e.g. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The figure below shows two titration curves: Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity. Titration Curve H2Co3.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve H2Co3 Naoh) added either to a strong acid (e.g. The volume of the titrant added. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Hcl) or to a weak acid (e.g. Figure 2 the titration curves of a strong base. The shape of a titration curve, a. Titration Curve H2Co3.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve H2Co3 The figure below shows two titration curves: Figure 2 the titration curves of a strong base. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant.. Titration Curve H2Co3.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Curve H2Co3 Hcl) or to a weak acid (e.g. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Our goal is to sketch the titration curve quickly,. The. Titration Curve H2Co3.
From chempedia.info
Titration carbonic acid Big Chemical Encyclopedia Titration Curve H2Co3 Naoh) added either to a strong acid (e.g. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. Figure 2 the titration curves of a strong base. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is.. Titration Curve H2Co3.
From www.slideserve.com
PPT Typical Applications of Neutralization Titrations Elemental Titration Curve H2Co3 Naoh) added either to a strong acid (e.g. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Figure 2 the titration curves of a strong base. Hcl) or to a weak acid (e.g. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$. Titration Curve H2Co3.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID5949370 Titration Curve H2Co3 Our goal is to sketch the titration curve quickly,. Figure 2 the titration curves of a strong base. Naoh) added either to a strong acid (e.g. The figure below shows two titration curves: Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The shape of a titration curve, a plot. Titration Curve H2Co3.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve H2Co3 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. The volume of the titrant added. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The shape of a titration curve, a plot of ph versus the amount of. Titration Curve H2Co3.
From www.bartleby.com
The composition diagram, or alpha plot, for the important acidbase Titration Curve H2Co3 Naoh) added either to a strong acid (e.g. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Our goal is to sketch the titration curve quickly,. The figure below shows two titration curves: Figure 2 the titration curves of a strong base. 1) for $\pu{10 ml}$. Titration Curve H2Co3.
From mungfali.com
Phosphate Titration Curve Titration Curve H2Co3 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Naoh) added either to a strong acid (e.g. The volume of the titrant added. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Our goal. Titration Curve H2Co3.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Ionic equilibrium deciding on end point Titration Curve H2Co3 The figure below shows two titration curves: The volume of the titrant added. Hcl) or to a weak acid (e.g. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. Titration Curve H2Co3.
From www.chegg.com
Solved curve of Carbonic acid (H2CO3) Titration Curve H2Co3 The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Figure 2 the titration curves of a strong base. The figure below shows. Titration Curve H2Co3.
From www.numerade.com
SOLVED Diprotic acids, such as carbonic acid (H2CO3), have two acidic Titration Curve H2Co3 Hcl) or to a weak acid (e.g. Naoh) added either to a strong acid (e.g. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Figure 2 the titration curves of a strong base. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$. Titration Curve H2Co3.
From www.numerade.com
SOLVED Diprotic acids, such as carbonic acid (H2CO3), have two acidic Titration Curve H2Co3 Hcl) or to a weak acid (e.g. The figure below shows two titration curves: Naoh) added either to a strong acid (e.g. The volume of the titrant added. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity of titrant. Vertical lines denote the equivalence points for titration of. Titration Curve H2Co3.
From www.numerade.com
SOLVED What is the area of the titration curve for carbonic acid Titration Curve H2Co3 Hcl) or to a weak acid (e.g. The figure below shows two titration curves: 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The volume of the titrant added.. Titration Curve H2Co3.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve H2Co3 Our goal is to sketch the titration curve quickly,. Naoh) added either to a strong acid (e.g. The figure below shows two titration curves: The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph. Titration Curve H2Co3.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Titration Curve H2Co3 Figure 2 the titration curves of a strong base. The volume of the titrant added. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10.. Titration Curve H2Co3.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve H2Co3 The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The figure below shows two titration curves: 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Hcl) or to a weak acid (e.g. The. Titration Curve H2Co3.
From www.trunorthink.com
Carbonate Chemistry Applied To The Productions Of Still Water Titration Curve H2Co3 Figure 2 the titration curves of a strong base. Our goal is to sketch the titration curve quickly,. 1) for $\pu{10 ml}$ of $\pu{0.1 m}$ carbonic acid, with $\pu{0.1 m}$ $\ce{naoh}$ titrant (black rising curve) and 2) for $\pu{10. Naoh) added either to a strong acid (e.g. The figure below shows two titration curves: The volume of the titrant added.. Titration Curve H2Co3.
From www.numerade.com
SOLVED 5. Draw a titration curve of HONH2 (Kb = 1.1x 10^8) with HCl Titration Curve H2Co3 Hcl) or to a weak acid (e.g. The figure below shows two titration curves: The volume of the titrant added. Vertical lines denote the equivalence points for titration of caustic alkalinity (ph co3), carbonate alkalinity (phhco3), and total alkalinity. The course of a titration can be followed by plotting the ph of the solution as a function of the quantity. Titration Curve H2Co3.