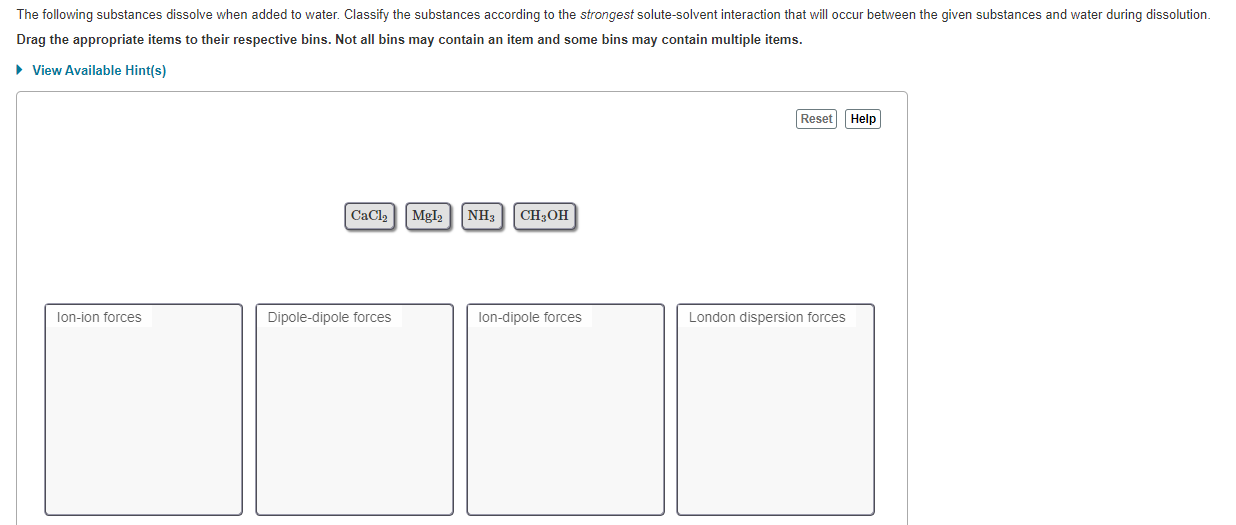
Which Of The Following Substances Will Dissolve In Water Check All That Apply . study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances dissolve when added to water. the following substances all dissolve to some extent in water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. Classify each as an electrolyte or a nonelectrolyte. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane.
from www.chegg.com
1 magnesium sulfate will dissolve in water because it is an ionic compound. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances dissolve when added to water. Classify each as an electrolyte or a nonelectrolyte. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances all dissolve to some extent in water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane.
Solved The following substances dissolve when added to
Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances all dissolve to some extent in water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances dissolve when added to water. Classify each as an electrolyte or a nonelectrolyte. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water.
From www.numerade.com
SOLVED Sort the following substances according to their behavior in aqueous solution. Assume Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances dissolve when added to water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances all dissolve to. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved 2. The following compounds dissolve readily in water. Which Of The Following Substances Will Dissolve In Water Check All That Apply magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. 1 magnesium sulfate will dissolve in water because it. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved The following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. Classify each as an electrolyte or a nonelectrolyte. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances dissolve when added to water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved Part C he following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. magnesium sulfate (mgso4) and sodium nitrate. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From byjus.com
Which of the given substances will dissolve in water? Which Of The Following Substances Will Dissolve In Water Check All That Apply the following substances all dissolve to some extent in water. the following substances dissolve when added to water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. 1 magnesium sulfate will dissolve in water because it is an ionic compound. study with quizlet and memorize flashcards containing terms like which. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. 1. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.numerade.com
SOLVED Five different substances are given to you to be dissolved in water. Which substances Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances all dissolve to some extent in water. Classify each as an electrolyte or a nonelectrolyte. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.nagwa.com
Question Video Identifying the Type of Substance Formed When a Nonmetal Oxide Dissolves in Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances dissolve when added to water. study. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.twinkl.com.ph
What is Dissolving? Answered Twinkl Teaching Wiki Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.numerade.com
SOLVED Text CH3OH H2O solution Use these sketches to answer the questions in the table below Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances dissolve when added to water. 1 magnesium sulfate. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved A small amount of sodium chloride NaCl is dissolved Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved The following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances dissolve when added to water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.cbsetuts.com
Which of the following substances are insoluble in water? CBSE Tuts Which Of The Following Substances Will Dissolve In Water Check All That Apply 1 magnesium sulfate will dissolve in water because it is an ionic compound. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate,. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From brainly.com
Which of the following substances most likely dissolve in water? Check all that apply •paraff Which Of The Following Substances Will Dissolve In Water Check All That Apply the following substances all dissolve to some extent in water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. the following substances dissolve when added to water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. study with quizlet and memorize flashcards containing terms like which of. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved te whether each of the following compounds dissolves Which Of The Following Substances Will Dissolve In Water Check All That Apply 1 magnesium sulfate will dissolve in water because it is an ionic compound. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances dissolve when added to water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. Classify each as an electrolyte or a. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved When the following substances dissolve in water, what Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances dissolve when added to water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved The following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply 1 magnesium sulfate will dissolve in water because it is an ionic compound. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances all dissolve to some extent in water. the following substances dissolve. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From cexsuajk.blob.core.windows.net
What Substance Dissolve In Water at Gail Whittaker blog Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances dissolve. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From slideplayer.com
Aim What are solubility factors? ppt download Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. 1 magnesium sulfate will dissolve in water because it is an ionic compound. Classify each as an electrolyte or a nonelectrolyte. the following substances all dissolve to some extent in water. the following substances dissolve when added to water. study with quizlet. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved When the following substances dissolve in water, what Which Of The Following Substances Will Dissolve In Water Check All That Apply magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. 1 magnesium sulfate will dissolve in water because it. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved The following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances dissolve. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved Classify the substances according to the strongest Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances dissolve when added to water. the following substances all dissolve to some extent in water. Classify each as an electrolyte or a nonelectrolyte. 1. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.numerade.com
SOLVED The following substances dissolve when added to water Classify the substances according Which Of The Following Substances Will Dissolve In Water Check All That Apply the following substances dissolve when added to water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances all dissolve to some extent in water. study with quizlet and memorize flashcards. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved Investigate the Special Properties of Water Question Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Which Of The Following Substances Will Dissolve In Water Check All That Apply the following substances all dissolve to some extent in water. the following substances dissolve when added to water. Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. study with quizlet and memorize flashcards containing terms like which of. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved When the following substances dissolve in water, what Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. Classify each as an electrolyte or a nonelectrolyte. the substances that will dissolve in water are magnesium sulfate, sodium. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Which Of The Following Substances Will Dissolve In Water Check All That Apply Classify each as an electrolyte or a nonelectrolyte. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID1977818 Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances all dissolve to some extent in water. Classify. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.youtube.com
water soluble substances and water insoluble substances YouTube Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the following substances all dissolve to some extent. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved The following substances dissolve when added to Which Of The Following Substances Will Dissolve In Water Check All That Apply the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. the following substances dissolve when added to water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.chegg.com
Solved substance classification (check all that apply) Which Of The Following Substances Will Dissolve In Water Check All That Apply study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. the following substances all dissolve to some extent in water. Classify each as an electrolyte or a nonelectrolyte. . Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Which Of The Following Substances Will Dissolve In Water Check All That Apply 1 magnesium sulfate will dissolve in water because it is an ionic compound. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. the substances that will dissolve in water are magnesium sulfate, sodium nitrate, and methane. the following substances all dissolve to some extent in water. study with quizlet and. Which Of The Following Substances Will Dissolve In Water Check All That Apply.
From www.numerade.com
SOLVED Which of the following substances would formulate a buffer solution? Select all that Which Of The Following Substances Will Dissolve In Water Check All That Apply the following substances dissolve when added to water. 1 magnesium sulfate will dissolve in water because it is an ionic compound. the following substances all dissolve to some extent in water. magnesium sulfate (mgso4) and sodium nitrate (nano3), being ionic compounds, will dissolve in water, whereas. Classify each as an electrolyte or a nonelectrolyte. study with. Which Of The Following Substances Will Dissolve In Water Check All That Apply.