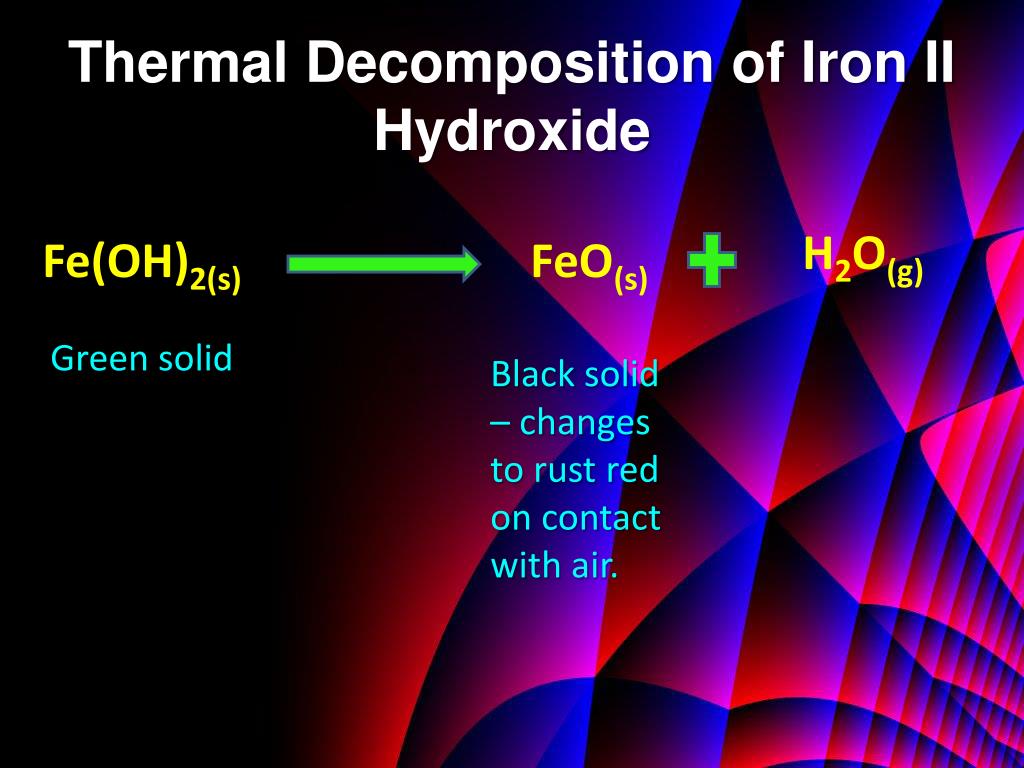
Heating Iron Iii Hydroxide . Iron (iii) hydroxide is most. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Synthesis of iron (iii) hydroxide. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. iron is very easily oxidized under alkaline conditions. The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water.
from www.slideserve.com
Iron (iii) hydroxide is most. The chemical equation for this reaction is: when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when heated, it decomposes, producing iron (iii) oxide and water. Synthesis of iron (iii) hydroxide. iron is very easily oxidized under alkaline conditions. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the.
PPT Thermal Reactions PowerPoint Presentation, free
Heating Iron Iii Hydroxide The chemical equation for this reaction is: Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. iron is very easily oxidized under alkaline conditions. when heated, it decomposes, producing iron (iii) oxide and water. The chemical equation for this reaction is: Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Iron (iii) hydroxide is most. Synthesis of iron (iii) hydroxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water.
From www.toppr.com
Iron III Hydroxide Formula Definition, Concepts and Examples Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. iron is very easily oxidized under alkaline conditions. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water.. Heating Iron Iii Hydroxide.
From chaitanyabio.com
IRON (III) HYDROXIDE POLYMALTOSE COMPLEX 34 Chaitanya Group of Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and. Heating Iron Iii Hydroxide.
From dir.indiamart.com
Iron Polymaltose Complex at Best Price in India Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. when heated, it decomposes, producing iron (iii) oxide and water. heating iron hydroxide results in. Heating Iron Iii Hydroxide.
From www.numerade.com
SOLVEDSolid iron(III) hydroxide is added to 625 \mathrm{mL} of 0.280 Heating Iron Iii Hydroxide when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. . Heating Iron Iii Hydroxide.
From www.numerade.com
SOLVED The reaction of iron (III) chloride and calcium hydroxide Heating Iron Iii Hydroxide The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water. iron is very easily oxidized under alkaline conditions. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. heating iron hydroxide results in the dehydration of the hydroxide to. Heating Iron Iii Hydroxide.
From www.tradeindia.com
Iron Iii Hydroxide Polymaltose Complex Syrup Specific Drug at Best Heating Iron Iii Hydroxide The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃. Heating Iron Iii Hydroxide.
From www.alamy.com
Iron (III) hydroxide precipitate formed by adding sodium hydroxide Heating Iron Iii Hydroxide Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. when heated, iron(iii) hydroxide decomposes to. Heating Iron Iii Hydroxide.
From studylib.net
SDS for Iron (III) Hydroxide, Alpha Heating Iron Iii Hydroxide iron is very easily oxidized under alkaline conditions. when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃. Heating Iron Iii Hydroxide.
From www.youtube.com
How to Write the Formula for Iron (III) hydroxide YouTube Heating Iron Iii Hydroxide iron is very easily oxidized under alkaline conditions. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxygen. Heating Iron Iii Hydroxide.
From chaitanyabio.com
IRON (III) HYDROXIDE POLYMALTOSE COMPLEX 34 Chaitanya Group of Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. iron is very easily oxidized under alkaline conditions. Iron (iii) hydroxide is most. when heated, it decomposes, producing iron (iii) oxide and water. The. Heating Iron Iii Hydroxide.
From www.youtube.com
How to find the molecular mass of Fe(OH)3 (Iron (III) Hydroxide) YouTube Heating Iron Iii Hydroxide when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: when you heat iron (iii) hydroxide, it breaks down to form. Heating Iron Iii Hydroxide.
From www.youtube.com
The Reaction Between Iron (III) Nitrate and Sodium Hydroxide YouTube Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. iron is very easily oxidized under alkaline conditions. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when. Heating Iron Iii Hydroxide.
From sciencephotogallery.com
Iron (iii) Hydroxide Precipitation by Science Photo Library Heating Iron Iii Hydroxide iron is very easily oxidized under alkaline conditions. Iron (iii) hydroxide is most. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when heated, it decomposes, producing iron. Heating Iron Iii Hydroxide.
From www.wbcil.com
Iron(III) Hydroxide Polymaltose Complex Supplier WBCIL Heating Iron Iii Hydroxide iron is very easily oxidized under alkaline conditions. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The chemical equation for this reaction is: Iron (iii) hydroxide is most. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Heating Iron Iii Hydroxide.
From www.reddit.com
Iron (III) Oxide precipitate from my lab today. I thought it looked Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. when heated, iron(iii) hydroxide decomposes to form. Heating Iron Iii Hydroxide.
From www.researchgate.net
TGA curves of reduction of iron(III) hydroxide in a hydrogen Heating Iron Iii Hydroxide when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. when. Heating Iron Iii Hydroxide.
From dxogvupte.blob.core.windows.net
Iron (Iii) Symbol And Charge at Rocky Molina blog Heating Iron Iii Hydroxide heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) hydroxide is most. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Synthesis of iron (iii) hydroxide. Oxygen in the air. Heating Iron Iii Hydroxide.
From www.researchgate.net
In situ heatflux calorimetry data a for iron hydroxide xerogels Heating Iron Iii Hydroxide Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Synthesis of iron (iii) hydroxide. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water. when heated, iron(iii) hydroxide decomposes to. Heating Iron Iii Hydroxide.
From testbook.com
Iron (III) Hydroxide Formula Its Structure, Preparation & Uses Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. when you heat iron (iii) hydroxide, it breaks down to form iron (iii). Heating Iron Iii Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image C027/9448 Science Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Synthesis of iron (iii) hydroxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. iron is very easily oxidized under alkaline conditions. heating iron hydroxide results in the dehydration of. Heating Iron Iii Hydroxide.
From www.shutterstock.com
Iron Iii Hydroxide Formula Handwritten Chemical Stock Illustration Heating Iron Iii Hydroxide when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The chemical equation for this reaction is: when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Synthesis of iron (iii) hydroxide. iron is very easily oxidized under alkaline conditions. heating iron hydroxide. Heating Iron Iii Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction iron iii hydroxide Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. Iron (iii) hydroxide is most. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. when heated, iron(iii) hydroxide. Heating Iron Iii Hydroxide.
From www.numerade.com
SOLVEDThe iron(III) ion content of a solution may be found by Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. The chemical equation for this reaction is: iron is very easily oxidized under alkaline conditions. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. heating iron hydroxide results in the dehydration of the hydroxide to form. Heating Iron Iii Hydroxide.
From www.youtube.com
The reaction of iron(III) solution with sodium hydroxide and ammonia Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. when heated, it decomposes, producing iron (iii) oxide and water. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water.. Heating Iron Iii Hydroxide.
From www.researchgate.net
(PDF) Sorption Properties of Freshly Precipitated Iron(III) Hydroxide Heating Iron Iii Hydroxide 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Synthesis of iron (iii) hydroxide. when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. iron is very easily oxidized under alkaline. Heating Iron Iii Hydroxide.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Heating Iron Iii Hydroxide iron is very easily oxidized under alkaline conditions. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Both methods result in the formation of. Heating Iron Iii Hydroxide.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Heating Iron Iii Hydroxide when heated, it decomposes, producing iron (iii) oxide and water. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate.. Heating Iron Iii Hydroxide.
From chaitanyabio.com
IRON (III) HYDROXIDE POLYMALTOSE COMPLEX 34 Chaitanya Group of Heating Iron Iii Hydroxide when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Synthesis of iron (iii) hydroxide. The chemical equation for this reaction. Heating Iron Iii Hydroxide.
From www.fishersci.se
Iron(III) hydroxide, 20 in H{2}O, nanoparticle dispersion, low pH, Heating Iron Iii Hydroxide Iron (iii) hydroxide is most. iron is very easily oxidized under alkaline conditions. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. when heated, it decomposes, producing iron (iii) oxide and water. The chemical equation for this reaction is: Oxygen in the air oxidizes the iron(ii). Heating Iron Iii Hydroxide.
From www.youtube.com
Iron III hydroxide precipitate, Chemistry Experiment YouTube Heating Iron Iii Hydroxide Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron (iii) hydroxide is most. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. iron is very easily oxidized under alkaline conditions. Synthesis of. Heating Iron Iii Hydroxide.
From www.shutterstock.com
Iron Iii Hydroxide Handwritten Chemical Formula ilustrações stock Heating Iron Iii Hydroxide Synthesis of iron (iii) hydroxide. iron is very easily oxidized under alkaline conditions. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The chemical equation for this reaction is: when heated, it decomposes, producing iron (iii) oxide and water. . Heating Iron Iii Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image A500/0413 Science Heating Iron Iii Hydroxide when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when heated, it decomposes, producing iron (iii) oxide and water. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate. Iron (iii) hydroxide is most. Synthesis of iron (iii) hydroxide. when heated, iron(iii) hydroxide decomposes to. Heating Iron Iii Hydroxide.
From www.numerade.com
SOLVED The thermal of iron(III) hydroxide to produce Heating Iron Iii Hydroxide The chemical equation for this reaction is: Iron (iii) hydroxide is most. Synthesis of iron (iii) hydroxide. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when heated, iron(iii) hydroxide decomposes to form iron(iii) oxide and water. Both methods. Heating Iron Iii Hydroxide.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube Heating Iron Iii Hydroxide heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Synthesis of iron (iii) hydroxide. when heated, it decomposes, producing iron (iii) oxide and water. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Oxygen in the air. Heating Iron Iii Hydroxide.
From www.slideserve.com
PPT Preview PowerPoint Presentation, free download ID6531641 Heating Iron Iii Hydroxide 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. when you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. iron is very easily oxidized under alkaline conditions. heating iron hydroxide results in the dehydration of the hydroxide to form iron oxide, and in this case, we know that the. Both methods result in the. Heating Iron Iii Hydroxide.