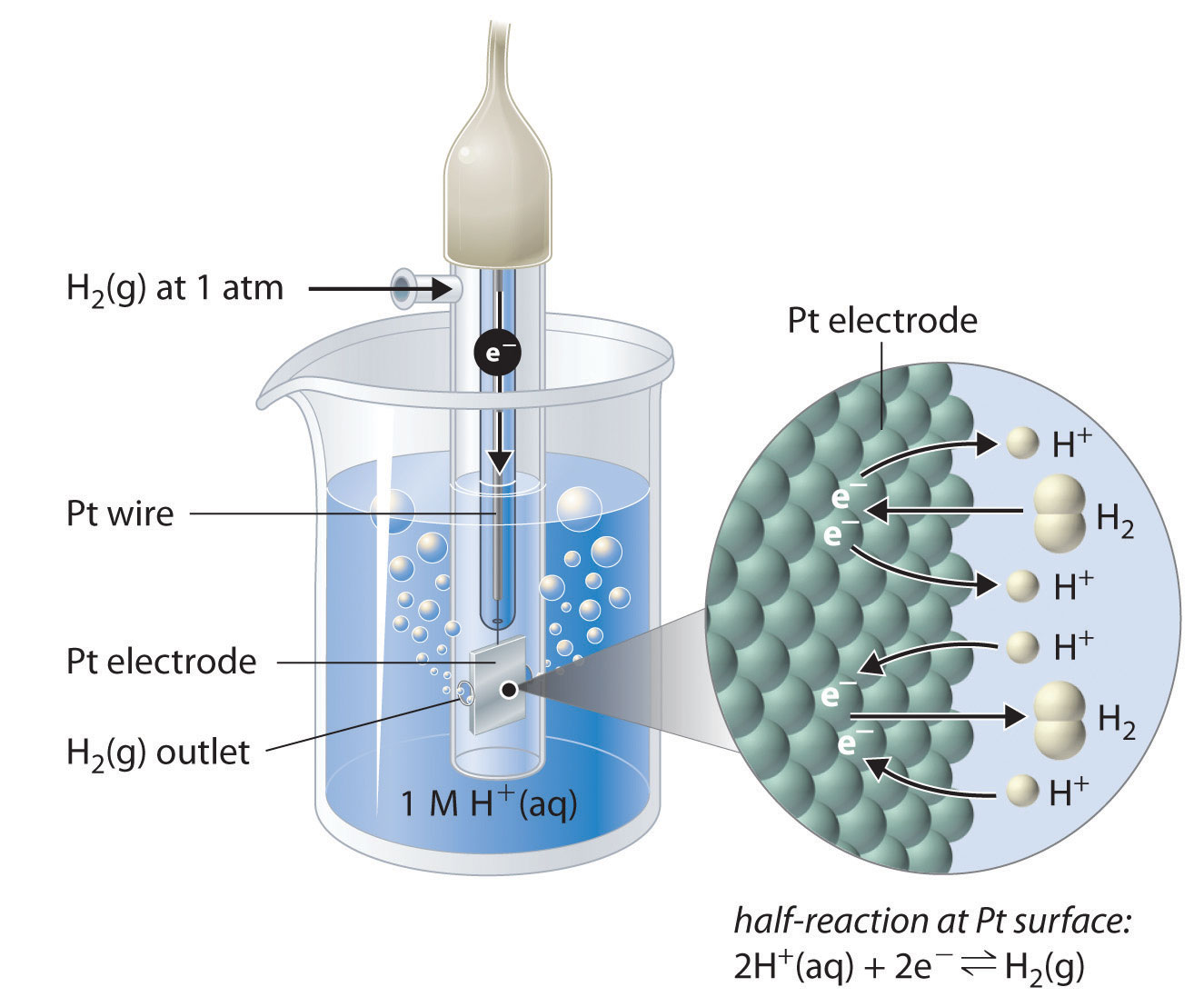
What Does Electrode Potential Mean . It also looks at how you go about. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. definition of electrode potential.
from chem.libretexts.org
To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. It also looks at how you go about. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. definition of electrode potential.
6.2 Standard Electrode Potentials Chemistry LibreTexts
What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. definition of electrode potential. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. It also looks at how you go about.
From www.youtube.com
Using Standard Electrode Potentials YouTube What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. definition of electrode potential. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the emf measured when a metal /. What Does Electrode Potential Mean.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog What Does Electrode Potential Mean It also looks at how you go about. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. definition of electrode potential. the emf measured when a. What Does Electrode Potential Mean.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Ch 14 Electrode Potentials PowerPoint Presentation, free What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the unit of electrode potential measured in volts, which is the amount. What Does Electrode Potential Mean.
From www.youtube.com
Introduction to Electrode Potentials YouTube What Does Electrode Potential Mean It also looks at how you go about. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. definition of electrode potential. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per.. What Does Electrode Potential Mean.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the emf measured when a metal / metal ion electrode is coupled to a hydrogen. What Does Electrode Potential Mean.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Does Electrode Potential Mean the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. definition of electrode potential. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell, measured in volts,. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. definition of electrode potential. It also looks at how you go about. this. What Does Electrode Potential Mean.
From app.pandai.org
Standard Electrode Potential What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the emf measured when a metal / metal ion electrode is coupled. What Does Electrode Potential Mean.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. definition of electrode potential. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It also looks at how you go about. the. What Does Electrode Potential Mean.
From quizlet.com
ELECTRODE POTENTIAL AND CELL Diagram Quizlet What Does Electrode Potential Mean the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. It also looks at how you go about. To determine the potential for the reduction of. What Does Electrode Potential Mean.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. To determine the potential for the reduction of zn 2+(aq) to zn (s). What Does Electrode Potential Mean.
From gamesmartz.com
Electrode Potential Definition & Image GameSmartz What Does Electrode Potential Mean definition of electrode potential. It also looks at how you go about. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of. What Does Electrode Potential Mean.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it. What Does Electrode Potential Mean.
From chemistry.stackexchange.com
electrochemistry Standard electrode potential for electrolytic vs What Does Electrode Potential Mean the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the emf measured when a metal / metal ion electrode is coupled to a hydrogen. What Does Electrode Potential Mean.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube What Does Electrode Potential Mean the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. definition of electrode potential. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It also looks at how you go about.. What Does Electrode Potential Mean.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry What Does Electrode Potential Mean this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. definition of electrode potential. the potential (e cell) of the cell, measured in volts, is the difference in electrical. What Does Electrode Potential Mean.
From chemistnotes.com
Single electrode potential Definition, origin, expression Chemistry What Does Electrode Potential Mean this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. definition of electrode potential. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. the emf measured when a metal / metal ion electrode is coupled to a hydrogen. What Does Electrode Potential Mean.
From www.britannica.com
Electricity Deriving, Electric Field, Potential Britannica What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of. What Does Electrode Potential Mean.
From mungfali.com
Electrode Potential Series What Does Electrode Potential Mean the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. definition of electrode potential. the potential (e cell) of. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemical Theory PowerPoint Presentation, free download What Does Electrode Potential Mean this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. definition of electrode potential. the emf measured when a metal / metal ion electrode is coupled to a hydrogen. What Does Electrode Potential Mean.
From www.youtube.com
What does electrode potential mean? YouTube What Does Electrode Potential Mean definition of electrode potential. It also looks at how you go about. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. To determine the potential for the. What Does Electrode Potential Mean.
From www.scribd.com
Standard Electrode Potentials What Does Electrode Potential Mean definition of electrode potential. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. It also looks at how you go about. the unit of electrode. What Does Electrode Potential Mean.
From in.pinterest.com
Electrode Potential Energy Definition, Formula and Example Potential What Does Electrode Potential Mean It also looks at how you go about. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. this page explains how to use. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. the unit of electrode potential measured in volts, which is the amount of energy required to move. What Does Electrode Potential Mean.
From www.youtube.com
CHEMISTRY A LEVEL ELECTRODE POTENTIAL YouTube What Does Electrode Potential Mean definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. the unit of electrode potential measured in volts, which is the amount of energy required. What Does Electrode Potential Mean.
From www.researchgate.net
Positive and negative electrode potential profiles from a NCM523/Gr What Does Electrode Potential Mean the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. definition of electrode potential. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. To determine the potential for the reduction of zn 2+(aq) to. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 What Does Electrode Potential Mean It also looks at how you go about. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. the potential (e cell). What Does Electrode Potential Mean.
From saylordotorg.github.io
Standard Potentials What Does Electrode Potential Mean It also looks at how you go about. the unit of electrode potential measured in volts, which is the amount of energy required to move one coulomb of electrons per. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell,. What Does Electrode Potential Mean.
From thechemistrynotes.com
Electrode Potential Types, Importance, and Applications What Does Electrode Potential Mean this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It also looks at how you go about. definition of electrode potential. To determine the potential for. What Does Electrode Potential Mean.
From www.youtube.com
Electrochemistry 9 Nernst Equation Find Electrode Potential or EMF What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. It also looks at how you go about. the emf measured when a metal / metal ion electrode is coupled. What Does Electrode Potential Mean.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 What Does Electrode Potential Mean definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. the unit of electrode potential measured in volts, which is the amount of energy required. What Does Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5368514 What Does Electrode Potential Mean definition of electrode potential. this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it. What Does Electrode Potential Mean.
From www.youtube.com
Difference between Oxidation potential and Reduction potential What Does Electrode Potential Mean To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell, measured in volts, is the difference in electrical potential between the two half. It also looks at how you go about. the emf measured when a metal / metal ion. What Does Electrode Potential Mean.
From question.pandai.org
Standard Electrode Potential What Does Electrode Potential Mean this page explains how to use redox potentials (electrode potentials) to predict the feasibility of redox reactions. definition of electrode potential. It also looks at how you go about. To determine the potential for the reduction of zn 2+(aq) to zn (s) we make it the cathode in the following. the potential (e cell) of the cell,. What Does Electrode Potential Mean.