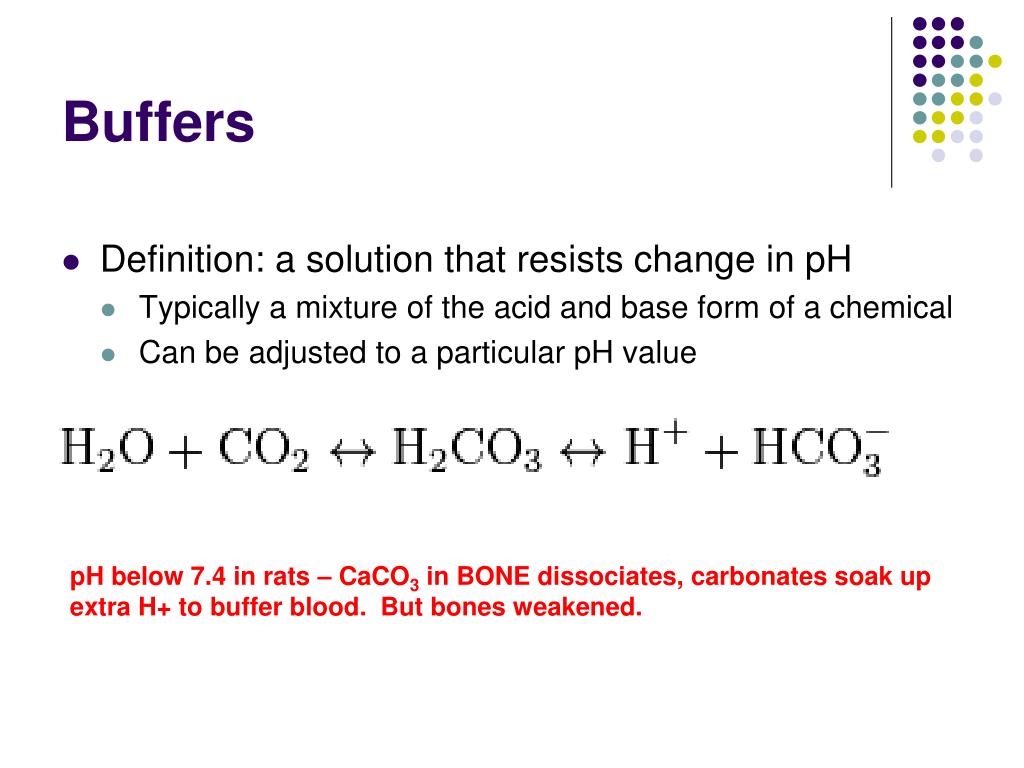
What Is The Difference Between Ph And Buffer Ph . the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. buffer ph will determine the quantity of liming material needed. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Buffer ph measures the “reserve acidity” and determines the. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution.
from www.slideserve.com
in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. Buffer ph measures the “reserve acidity” and determines the. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution.
PPT pH and Buffers PowerPoint Presentation, free download ID2948821
What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine the quantity of liming material needed. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. Buffer ph measures the “reserve acidity” and determines the.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. buffer ph will determine the quantity of liming material needed. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co. What Is The Difference Between Ph And Buffer Ph.
From www.slideshare.net
Buffer system What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately,. What Is The Difference Between Ph And Buffer Ph.
From content.byui.edu
Acids, Bases, pH and Buffers What Is The Difference Between Ph And Buffer Ph while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine the quantity of liming. What Is The Difference Between Ph And Buffer Ph.
From exyevaapo.blob.core.windows.net
What Does Ph Buffer Do In A Pool at David Crabtree blog What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the. What Is The Difference Between Ph And Buffer Ph.
From www.studypool.com
SOLUTION 3 water ph and buffer ppt Studypool What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph. What Is The Difference Between Ph And Buffer Ph.
From giovanna-has-townsend.blogspot.com
What Is the Unique Characterization of a Ph Buffer GiovannahasTownsend What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. Buffer ph measures the “reserve acidity” and determines the. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of. What Is The Difference Between Ph And Buffer Ph.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Is The Difference Between Ph And Buffer Ph while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. buffer ph will determine the quantity of liming material needed. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately,. What Is The Difference Between Ph And Buffer Ph.
From studylib.net
pH and Buffers What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate. What Is The Difference Between Ph And Buffer Ph.
From www.slideshare.net
Ph and buffer What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine. What Is The Difference Between Ph And Buffer Ph.
From kuncijawabanpdf.blogspot.com
Soal Dapar Ph 7 4 Kunci Jawaban What Is The Difference Between Ph And Buffer Ph in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. the science. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and,. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT Chapter 2 The Chemistry of Life PowerPoint Presentation, free What Is The Difference Between Ph And Buffer Ph while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. Buffer ph measures the “reserve acidity”. What Is The Difference Between Ph And Buffer Ph.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and,. What Is The Difference Between Ph And Buffer Ph.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. buffer. What Is The Difference Between Ph And Buffer Ph.
From www.researchgate.net
Comparison of reactions in sodium phosphate buffer (pH 7.0) and What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity. What Is The Difference Between Ph And Buffer Ph.
From preclaboratories.com
pH and Buffers How Buffer Solutions Maintain pH Precision Laboratories What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. in general, the soil. What Is The Difference Between Ph And Buffer Ph.
From www.youtube.com
pH and buffers overview for biology YouTube What Is The Difference Between Ph And Buffer Ph while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. Buffer ph measures the “reserve acidity” and determines. What Is The Difference Between Ph And Buffer Ph.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube What Is The Difference Between Ph And Buffer Ph while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. Buffer ph measures the “reserve acidity” and determines the. buffer ph will determine the quantity of liming material needed. the science behind how a buffer functions to maintain ph, however, is complex, and here. What Is The Difference Between Ph And Buffer Ph.
From exyevaapo.blob.core.windows.net
What Does Ph Buffer Do In A Pool at David Crabtree blog What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine. What Is The Difference Between Ph And Buffer Ph.
From www.shutterstock.com
Bicarbonate Buffer Ph Scale Stock Vector (Royalty Free) 1839896080 What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. buffer ph will determine the quantity of liming material needed. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co. What Is The Difference Between Ph And Buffer Ph.
From www.scribd.com
pH and Buffers Acid Buffer Solution What Is The Difference Between Ph And Buffer Ph in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. buffer ph will determine the quantity of liming material needed. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h. What Is The Difference Between Ph And Buffer Ph.
From www.slideshare.net
Electrophoresis What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co. What Is The Difference Between Ph And Buffer Ph.
From chemistrycalc.com
Buffer pH Calculator Tool to find pH of a Buffer Solution What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of. What Is The Difference Between Ph And Buffer Ph.
From www.askdifference.com
pH vs. Buffer — What’s the Difference? What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Buffer ph measures the “reserve acidity” and determines the. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and,. What Is The Difference Between Ph And Buffer Ph.
From www.researchgate.net
pH of distilled water and pH 7 buffer solution at various temperatures What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. while buffer refers to. What Is The Difference Between Ph And Buffer Ph.
From mmerevise.co.uk
pH Curves Questions and Revision MME What Is The Difference Between Ph And Buffer Ph in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Buffer ph measures. What Is The Difference Between Ph And Buffer Ph.
From courses.lumenlearning.com
pH, Buffers, Acids, and Bases Introduction to Chemistry What Is The Difference Between Ph And Buffer Ph Buffer ph measures the “reserve acidity” and determines the. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. What Is The Difference Between Ph And Buffer Ph.
From exydsrovs.blob.core.windows.net
How To Use Buffer Solution Ph at Clint Stacey blog What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. Buffer ph. What Is The Difference Between Ph And Buffer Ph.
From www.youtube.com
Buffers and pH Biology YouTube What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph, ph is a measure of the acidity or alkalinity of a solution. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately,. What Is The Difference Between Ph And Buffer Ph.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID What Is The Difference Between Ph And Buffer Ph the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical concepts behind it, starting with a. Buffer ph measures the “reserve acidity” and determines the. buffer ph will determine the quantity of liming material needed. while buffer refers to a solution that resists changes in ph,. What Is The Difference Between Ph And Buffer Ph.
From www.reddit.com
Is it just me or does the bottom right graph contradict the What Is The Difference Between Ph And Buffer Ph buffer ph will determine the quantity of liming material needed. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. the science behind how a buffer functions to maintain ph, however, is complex, and here we will learn about the theoretical. What Is The Difference Between Ph And Buffer Ph.
From www.differencebetween.com
Difference Between pH and Buffer Compare the Difference Between What Is The Difference Between Ph And Buffer Ph vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. in general, the soil ph tells a farmer if they have an acidic (ph < 7) or alkaline (ph > 7) soil and, ultimately, if they need to lime. while buffer. What Is The Difference Between Ph And Buffer Ph.