Iron Element Number Of Protons Neutrons And Electrons . The number of neutrons in an atom can be determined by the difference between the atomic mass and. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Protons and neutrons in iron. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Iron is a chemical element with atomic number 26. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell.
from mybios.me
Determine the number of protons and electrons. Iron is a chemical element with atomic number 26. Describe the locations, charges, and masses of the three main subatomic particles. Protons and neutrons in iron. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The number of neutrons in an atom can be determined by the difference between the atomic mass and. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to:
Using The Periodic Table To Determine Protons Neutrons And Electrons
Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Protons and neutrons in iron. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Determine the number of protons and electrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Describe the locations, charges, and masses of the three main subatomic particles. The number of neutrons in an atom can be determined by the difference between the atomic mass and. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Iron is a chemical element with atomic number 26.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Describe the locations, charges, and masses of the three main subatomic particles.. Iron Element Number Of Protons Neutrons And Electrons.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The number of neutrons in. Iron Element Number Of Protons Neutrons And Electrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The number of. Iron Element Number Of Protons Neutrons And Electrons.
From azchemistry.com
Proton, Electron, Neutron Definition Formula Application Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Protons and neutrons in iron. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The number of neutrons in an atom can. Iron Element Number Of Protons Neutrons And Electrons.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. We know that the mass number (a) = number of protons +. Iron Element Number Of Protons Neutrons And Electrons.
From awesomehome.co
Periodic Table Of Elements List With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is. Iron Element Number Of Protons Neutrons And Electrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Protons. Iron Element Number Of Protons Neutrons And Electrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total. Iron Element Number Of Protons Neutrons And Electrons.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Describe the locations, charges, and masses of the three main subatomic particles. Iron is a chemical. Iron Element Number Of Protons Neutrons And Electrons.
From brainly.ph
Complete the table below, write the atomic number, number of protons Iron Element Number Of Protons Neutrons And Electrons The number of neutrons in an atom can be determined by the difference between the atomic mass and. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. We. Iron Element Number Of Protons Neutrons And Electrons.
From mybios.me
Using The Periodic Table To Determine Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Determine the number of protons and electrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element with atomic number 26. Protons and neutrons in iron. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus.. Iron Element Number Of Protons Neutrons And Electrons.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons Iron Element Number Of Protons Neutrons And Electrons We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Protons and neutrons in iron. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. 112 rows protons, neutrons. Iron Element Number Of Protons Neutrons And Electrons.
From mybios.me
Using The Periodic Table To Determine Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Iron is a chemical element with atomic number 26. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Determine the number of. Iron Element Number Of Protons Neutrons And Electrons.
From www.periodictableprintable.com
How To Calculate The Number Of Protons Neutrons And Electrons Using The Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Determine the number of protons and electrons. We know. Iron Element Number Of Protons Neutrons And Electrons.
From www.slideserve.com
PPT How to find the number of protons, neutrons, & electrons for an Iron Element Number Of Protons Neutrons And Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Describe the locations, charges, and masses of the three main subatomic particles. We know that the mass number (a) =. Iron Element Number Of Protons Neutrons And Electrons.
From physicsteacher.in
The atoms of the first 20 elements in the periodic table Iron Element Number Of Protons Neutrons And Electrons We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Determine the number of protons and electrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Iron is a chemical element with. Iron Element Number Of Protons Neutrons And Electrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Iron is a chemical element with atomic number 26. Determine the number of protons and electrons. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Describe the locations, charges,. Iron Element Number Of Protons Neutrons And Electrons.
From www.periodictableprintable.com
Periodic Table Element Proton Neutron Electron Periodic Table Printable Iron Element Number Of Protons Neutrons And Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Iron is a chemical element with atomic number 26. We know that the mass number (a) = number of. Iron Element Number Of Protons Neutrons And Electrons.
From utedzz.blogspot.com
Periodic Table Of Elements List With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Determine the number of protons and electrons. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Describe the locations, charges, and masses of the three main subatomic particles. The number. Iron Element Number Of Protons Neutrons And Electrons.
From mavink.com
Protons In The Periodic Table Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26. Iron Element Number Of Protons Neutrons And Electrons.
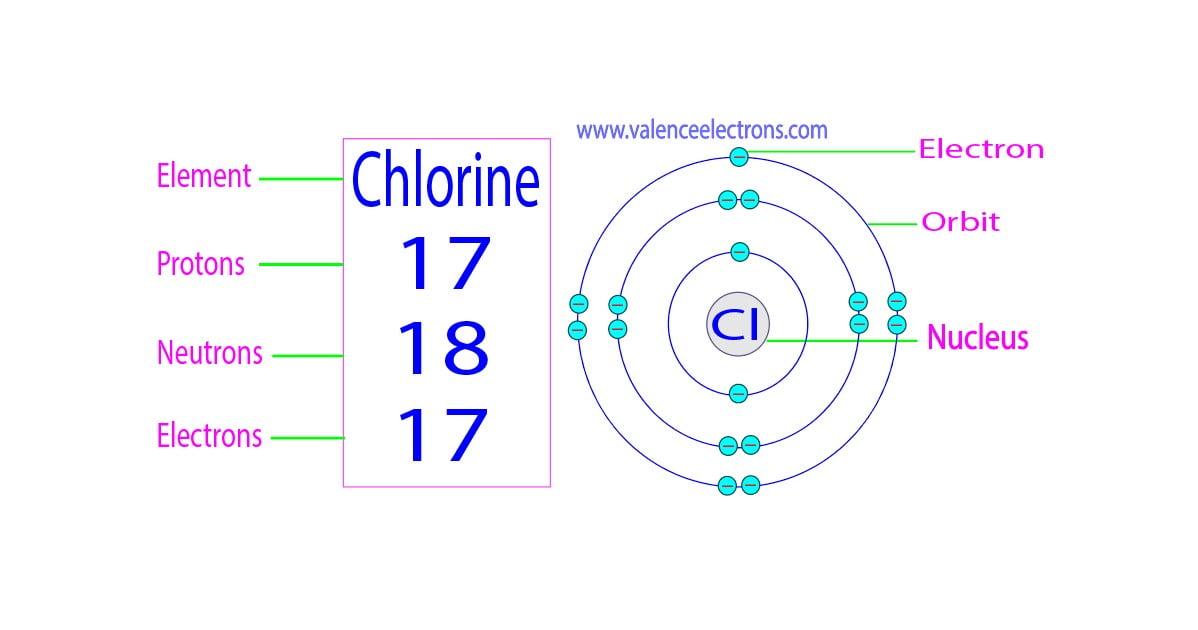
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Iron is a chemical element with atomic number 26. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Iron is a chemical element of the periodic table with chemical symbol fe and. Iron Element Number Of Protons Neutrons And Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Element Number Of Protons Neutrons And Electrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Determine the number of protons and electrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Iron is a chemical element with atomic number 26. Iron. Iron Element Number Of Protons Neutrons And Electrons.
From www.vrogue.co
Atomic Nucleus Proton Electron Chemistry Atomo Png Pn vrogue.co Iron Element Number Of Protons Neutrons And Electrons The number of neutrons in an atom can be determined by the difference between the atomic mass and. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight. Iron Element Number Of Protons Neutrons And Electrons.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Protons Neutrons Electrons Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is a chemical element with atomic number 26. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. 112 rows protons, neutrons and electrons of. Iron Element Number Of Protons Neutrons And Electrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Describe the locations, charges, and masses of. Iron Element Number Of Protons Neutrons And Electrons.
From ar.inspiredpencil.com
Proton Neutron Electron Periodic Table Iron Element Number Of Protons Neutrons And Electrons The number of neutrons in an atom can be determined by the difference between the atomic mass and. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element with atomic number 26. 112 rows protons, neutrons and electrons of all elements are mentioned in. Iron Element Number Of Protons Neutrons And Electrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Iron Element Number Of Protons Neutrons And Electrons The number of neutrons in an atom can be determined by the difference between the atomic mass and. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol. Iron Element Number Of Protons Neutrons And Electrons.
From studylib.net
atom Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26. Protons and neutrons in iron. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number. Iron Element Number Of Protons Neutrons And Electrons.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons Iron Element Number Of Protons Neutrons And Electrons Determine the number of protons and electrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. We know that the mass number (a) = number of. Iron Element Number Of Protons Neutrons And Electrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Element Number Of Protons Neutrons And Electrons Determine the number of protons and electrons. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Protons and neutrons in iron. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. We know that the mass number (a) = number. Iron Element Number Of Protons Neutrons And Electrons.
From sciencing.com
How to Find the Neutrons in the Periodic Table Sciencing Iron Element Number Of Protons Neutrons And Electrons Describe the locations, charges, and masses of the three main subatomic particles. Protons and neutrons in iron. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Iron is a chemical. Iron Element Number Of Protons Neutrons And Electrons.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry Iron Element Number Of Protons Neutrons And Electrons The number of neutrons in an atom can be determined by the difference between the atomic mass and. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Describe the locations, charges, and masses of the three main subatomic particles. Iron is a chemical element. Iron Element Number Of Protons Neutrons And Electrons.
From awesomehome.co
Using The Periodic Table To Determine Protons Neutrons And Electrons Iron Element Number Of Protons Neutrons And Electrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Describe the locations, charges, and masses of the three main subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Iron is a chemical element. Iron Element Number Of Protons Neutrons And Electrons.
From slidetodoc.com
Introduction to Basic Chemistry Protons Neutrons Electrons and Iron Element Number Of Protons Neutrons And Electrons Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Describe the locations, charges, and masses of the three main subatomic particles. Iron is a chemical element with atomic number 26. Iron is a. Iron Element Number Of Protons Neutrons And Electrons.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Iron Element Number Of Protons Neutrons And Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The number of neutrons in an atom can be determined by the difference between the atomic mass and. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol. Iron Element Number Of Protons Neutrons And Electrons.