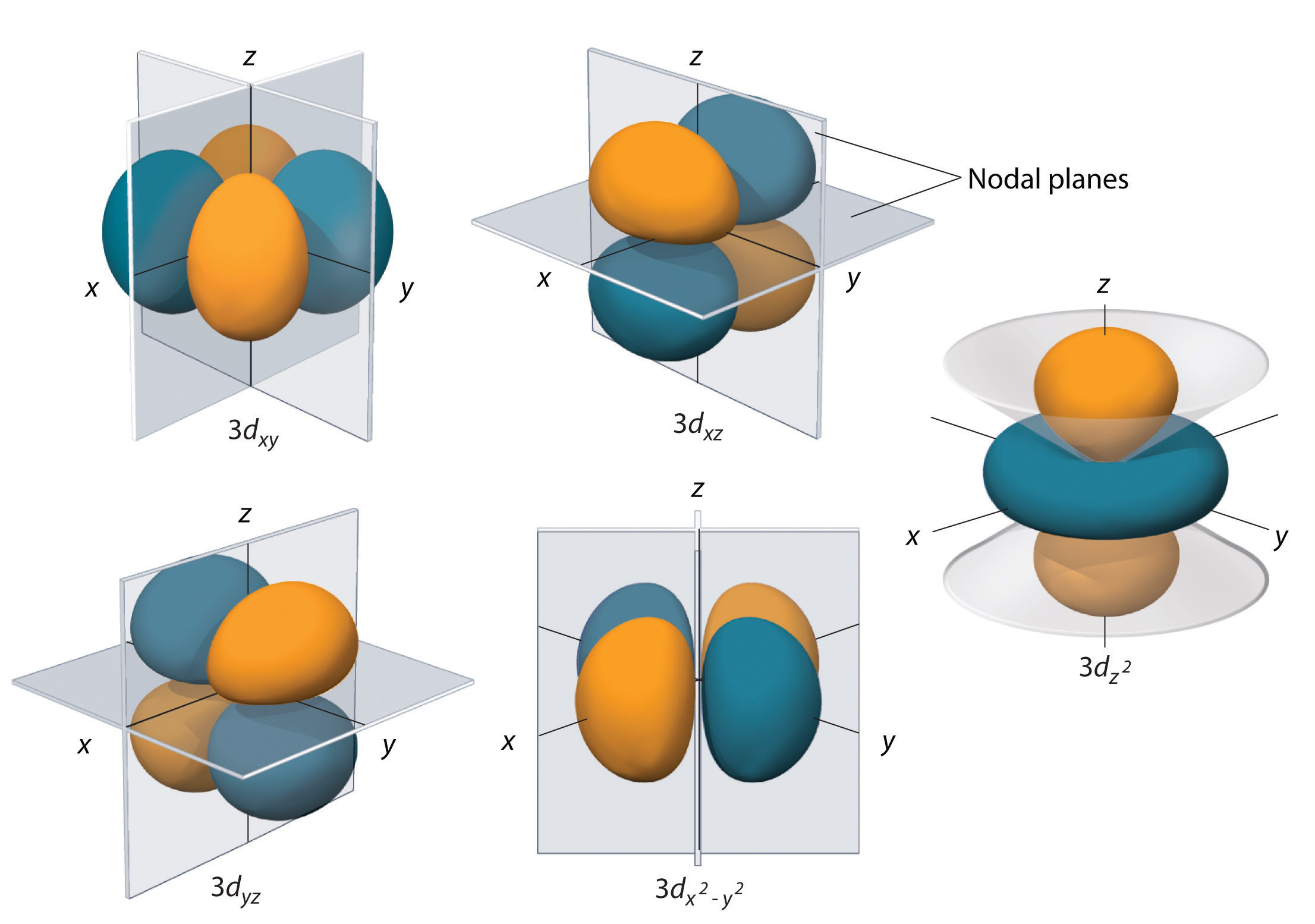
What Is Orbital And Shell . The shells of an atom can be thought of concentric circles. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. Check out the next lesson and practice what you’re. another name for the principal quantum number is the shell number. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom.
from chem.libretexts.org
orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. another name for the principal quantum number is the shell number. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. The shells of an atom can be thought of concentric circles. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. Check out the next lesson and practice what you’re. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams.
6.6 3D Representation of Orbitals Chemistry LibreTexts
What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. Check out the next lesson and practice what you’re. The shells of an atom can be thought of concentric circles. another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is Orbital And Shell another name for the principal quantum number is the shell number. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. Check out the next lesson and practice what you’re. the orbital of p subshell all have two lobes of electron density pointing along each of the axes.. What Is Orbital And Shell.
From www.cyberphysics.co.uk
Physics revision GCSE and A Level Physics Revision Cyberphysics What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. The shells of an atom can be thought of. What Is Orbital And Shell.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. another name for the principal quantum number is the shell number. different shells contain different. What Is Orbital And Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is Orbital And Shell The shells of an atom can be thought of concentric circles. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. the orbital of p subshell all. What Is Orbital And Shell.
From www.youtube.com
Orbits, Shells, Subshells and Orbitals CSIR Life Sciences CYTOLOGY What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. The shells of an atom can be thought of concentric circles. Check out the next lesson and practice what you’re. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. . What Is Orbital And Shell.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the. What Is Orbital And Shell.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. Check out the next lesson and practice what you’re. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. another name for the principal quantum number. What Is Orbital And Shell.
From lavelle.chem.ucla.edu
subshells and orbitals CHEMISTRY COMMUNITY What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. another name for the principal quantum number is the shell number. The shells of an atom. What Is Orbital And Shell.
From jaroslavlachky.sk
FÓRUM Jaroslav Lachký What Is Orbital And Shell another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and. What Is Orbital And Shell.
From www.youtube.com
ORBIT vs ORBITAL electrons 3min Quick short differences YouTube What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. Check out the next lesson and practice what you’re. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. revision notes on 1.1.4 shells and. What Is Orbital And Shell.
From www.britannica.com
Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica What Is Orbital And Shell another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. orbitals within a. What Is Orbital And Shell.
From www.vrogue.co
How Many Orbitals Are There In D Subshell Chemistry S vrogue.co What Is Orbital And Shell The shells of an atom can be thought of concentric circles. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams.. What Is Orbital And Shell.
From antranik.org
Electrons shells and orbitals What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction. What Is Orbital And Shell.
From chem.libretexts.org
6.6 The Shapes of Atomic Orbitals Chemistry LibreTexts What Is Orbital And Shell different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. The shells of an atom can be thought of concentric circles. Check out the next lesson and practice what you’re. another name for the principal quantum number is the shell number. the orbital of p subshell. What Is Orbital And Shell.
From guidelibsolidarity.z21.web.core.windows.net
Orbital Diagram Chart What Is Orbital And Shell the orbital of p subshell all have two lobes of electron density pointing along each of the axes. another name for the principal quantum number is the shell number. The shells of an atom can be thought of concentric circles. Check out the next lesson and practice what you’re. revision notes on 1.1.4 shells and orbitals for. What Is Orbital And Shell.
From www.youtube.com
Shells, Subshells, and Orbitals, Oh My! YouTube What Is Orbital And Shell The shells of an atom can be thought of concentric circles. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. orbitals. What Is Orbital And Shell.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. another name for the principal quantum number is the shell number. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. The shells. What Is Orbital And Shell.
From www.e-magnetica.pl
[Encyclopedia What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. the orbital of p subshell all have two lobes of electron density pointing. What Is Orbital And Shell.
From brainly.in
What is shell subshells and orbitals? with examples and diagrams What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. Check out the next lesson and practice what you’re. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. another name for the principal quantum number is. What Is Orbital And Shell.
From polizhuge.weebly.com
Atomic orbitals explained polizhuge What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. another name for the principal quantum number is the shell number. revision notes. What Is Orbital And Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is Orbital And Shell different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. The shells of an atom can be thought of concentric circles. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. another name for the principal quantum number. What Is Orbital And Shell.
From chemistry.stackexchange.com
quantum chemistry How do 1s and 2p orbitals overlap? Chemistry What Is Orbital And Shell while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. Check out the next lesson and practice what you’re. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the. What Is Orbital And Shell.
From www.peoi.org
Chapter 8 Section B Quantum Numbers for Electrons What Is Orbital And Shell The shells of an atom can be thought of concentric circles. another name for the principal quantum number is the shell number. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. different shells contain different numbers and kinds of orbitals, and each. What Is Orbital And Shell.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative What Is Orbital And Shell another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. while the concepts of electron shells. What Is Orbital And Shell.
From exooogfvb.blob.core.windows.net
What Is The Electron Distribution In Shells at Ruby Lehmann blog What Is Orbital And Shell orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. Check out the next lesson and practice what you’re. another name for the principal quantum number is the shell number. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry. What Is Orbital And Shell.
From brainly.in
differentiate between shell subshell and orbitals with the help of What Is Orbital And Shell while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. orbitals within a shell are divided into subshells that have the same value of the. What Is Orbital And Shell.
From icdsc.org
How many electrons are in each shell including 3p orbitals What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. Check out the next lesson and practice what you’re. while the concepts of electron shells. What Is Orbital And Shell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is Orbital And Shell The shells of an atom can be thought of concentric circles. while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. another name for the principal quantum number is the shell number. Check out the next lesson and practice what you’re. orbitals within. What Is Orbital And Shell.
From bilifforyou.blogspot.com
Why are the orbitals shells called s, p, d, f, etc.? Is there a reason? What Is Orbital And Shell another name for the principal quantum number is the shell number. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. while the concepts of electron shells and orbitals. What Is Orbital And Shell.
From socratic.org
What is the difference between electron shells and electron orbitals What Is Orbital And Shell The shells of an atom can be thought of concentric circles. another name for the principal quantum number is the shell number. orbitals within a shell are divided into subshells that have the same value of the angular quantum number $l$. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can. What Is Orbital And Shell.
From www.pinterest.com
Atoms, shells ,Subshells and Orbitals What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. another name for the principal quantum number is the shell number. Check out the next lesson and practice what you’re. different shells contain different numbers and kinds of orbitals, and each orbital within a. What Is Orbital And Shell.
From circuitdiagramhinge.z5.web.core.windows.net
Electron Orbital Diagram Calculator What Is Orbital And Shell another name for the principal quantum number is the shell number. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. orbitals within a shell are divided. What Is Orbital And Shell.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent What Is Orbital And Shell Check out the next lesson and practice what you’re. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The shells of an atom can be thought of concentric circles. while the concepts of electron shells and orbitals are closely related, orbitals provide a more. What Is Orbital And Shell.
From socratic.org
Which are the orbitals(s,p,d,f) have center of symmetry? Socratic What Is Orbital And Shell revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. The shells of an atom can be thought of concentric circles. another name for the principal. What Is Orbital And Shell.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is Orbital And Shell while the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom. Check out the next lesson and practice what you’re. the orbital of p subshell all have two lobes of electron density pointing along each of the axes. orbitals within a shell are divided. What Is Orbital And Shell.