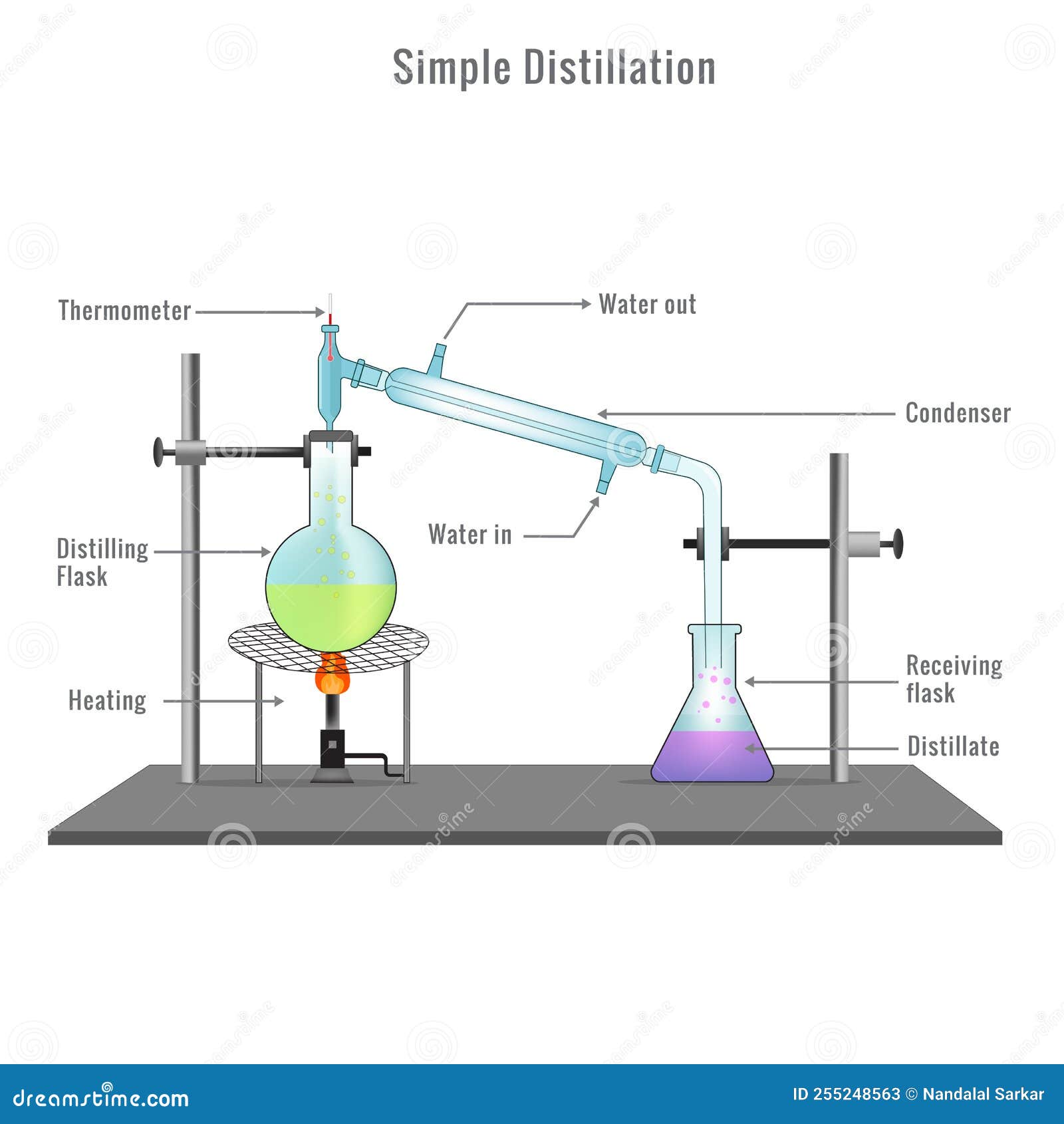
Explain Simple Distillation With Diagram . There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Simple distillation is what you would use to get, for example, pure water from sea water. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Learn more in this ks3 chemistry guide from. Distillation separates a soluble substance from its solvent based on differences in boiling points. Explain the role of the lever rule in fractional. There is only one liquid present. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation requires multiple pieces of. If you boil sea water,.
from www.dreamstime.com
Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation is a separation technique used to remove a solvent from a mixture and keep it. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Simple distillation is what you would use to get, for example, pure water from sea water. Distillation requires multiple pieces of. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from. There is only one liquid present. Distillation separates a soluble substance from its solvent based on differences in boiling points. If you boil sea water,.
Simple Distillation Apparatus Diagram with Full Process Vector
Explain Simple Distillation With Diagram Simple distillation is a separation technique that capitalizes on differences in boiling points to. Learn more in this ks3 chemistry guide from. Distillation requires multiple pieces of. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. If you boil sea water,. Distillation separates a soluble substance from its solvent based on differences in boiling points. There is only one liquid present. Explain the role of the lever rule in fractional. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Simple distillation is what you would use to get, for example, pure water from sea water. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation.
From myphys.co.uk
simple distillation Mychem Explain Simple Distillation With Diagram Distillation separates a soluble substance from its solvent based on differences in boiling points. Simple distillation is what you would use to get, for example, pure water from sea water. Distillation requires multiple pieces of. Distillation is a separation technique used to remove a solvent from a mixture and keep it. If you boil sea water,. There are different ways. Explain Simple Distillation With Diagram.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Explain Simple Distillation With Diagram Distillation separates a soluble substance from its solvent based on differences in boiling points. Learn more in this ks3 chemistry guide from. Distillation requires multiple pieces of. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. If you boil sea water,. There is only one liquid present. Simple distillation is a separation technique that capitalizes. Explain Simple Distillation With Diagram.
From ar.inspiredpencil.com
Distillation Diagram For Kids Explain Simple Distillation With Diagram If you boil sea water,. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation separates a soluble substance from its solvent based on differences in boiling points. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Learn more in this ks3 chemistry guide from. Explain the role of the. Explain Simple Distillation With Diagram.
From brainly.in
Draw the neat labelled digram of simple distillation Brainly.in Explain Simple Distillation With Diagram Simple distillation is what you would use to get, for example, pure water from sea water. Distillation is a separation technique used to remove a solvent from a mixture and keep it. There is only one liquid present. If you boil sea water,. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from.. Explain Simple Distillation With Diagram.
From www.vecteezy.com
Distillation process diagram for education 3303824 Vector Art at Vecteezy Explain Simple Distillation With Diagram Simple distillation is a separation technique that capitalizes on differences in boiling points to. There is only one liquid present. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Explain the role of the lever rule in fractional. Distillation requires multiple pieces of. Learn more in this ks3 chemistry guide from. There are. Explain Simple Distillation With Diagram.
From mavink.com
Distillation Apparatus Labelled Diagram Explain Simple Distillation With Diagram There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry guide from. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Simple distillation is what you would use to get, for example, pure water from sea water. There is only one liquid present. Distillation. Explain Simple Distillation With Diagram.
From mavink.com
Labelled Diagram Of Distillation Explain Simple Distillation With Diagram Distillation is a separation technique used to remove a solvent from a mixture and keep it. Explain the role of the lever rule in fractional. There is only one liquid present. Distillation requires multiple pieces of. Learn more in this ks3 chemistry guide from. Distillation separates a soluble substance from its solvent based on differences in boiling points. Simple distillation. Explain Simple Distillation With Diagram.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Explain Simple Distillation With Diagram Learn more in this ks3 chemistry guide from. Distillation separates a soluble substance from its solvent based on differences in boiling points. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Distillation requires multiple pieces of. There is only one liquid present. Simple distillation is a separation technique that capitalizes on differences in boiling points. Explain Simple Distillation With Diagram.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Explain Simple Distillation With Diagram Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation separates a soluble substance from its solvent based on differences in boiling points. Learn more in this ks3 chemistry guide from. Explain the role of the lever rule in fractional. Simple distillation is a separation technique that capitalizes on differences in boiling points. Explain Simple Distillation With Diagram.
From en.wikipedia.org
Distillation Wikipedia Explain Simple Distillation With Diagram There is only one liquid present. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation requires multiple pieces of. If you boil sea water,. Distillation separates a soluble substance from its solvent based on differences in boiling points. Simple distillation is a separation technique that capitalizes on differences in boiling points to.. Explain Simple Distillation With Diagram.
From zamcopter.web.fc2.com
SIMPLE DISTILLATION Explain Simple Distillation With Diagram Simple distillation is what you would use to get, for example, pure water from sea water. There is only one liquid present. Learn more in this ks3 chemistry guide from. Distillation is a separation technique used to remove a solvent from a mixture and keep it. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. There. Explain Simple Distillation With Diagram.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Explain Simple Distillation With Diagram Distillation requires multiple pieces of. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Explain the role of the lever rule in fractional. If you boil sea water,. There is only one liquid present. Simple distillation is what you would use to get, for example, pure water from sea water. Distillation is a separation technique. Explain Simple Distillation With Diagram.
From medium.com
‘Ultrapure Water and Distilled Water — What is the Difference? by Explain Simple Distillation With Diagram There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Learn more in this ks3 chemistry guide from. If you boil sea water,. Simple distillation is what you would use to get, for example, pure water from sea water. Simple distillation is a. Explain Simple Distillation With Diagram.
From www.youtube.com
Animated Water Distillation process diagram in PowerPoint / Chemistry Explain Simple Distillation With Diagram Simple distillation is what you would use to get, for example, pure water from sea water. If you boil sea water,. Simple distillation is a separation technique that capitalizes on differences in boiling points to. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. There are different ways to separate mixtures, such as filtration, crystallisation, simple. Explain Simple Distillation With Diagram.
From www.shutterstock.com
diagramme de distillation simple en chimie image vectorielle de stock Explain Simple Distillation With Diagram There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation separates a soluble substance from its solvent based on differences in boiling points. There is only one liquid present. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from. If you boil sea water,. Distillation is a separation technique. Explain Simple Distillation With Diagram.
From mungfali.com
Diagram Of Distillation Explain Simple Distillation With Diagram Explain the role of the lever rule in fractional. If you boil sea water,. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation requires multiple pieces of. There is only one liquid present. Distillation separates a soluble substance from its solvent based on differences in boiling points. There are different ways to separate mixtures,. Explain Simple Distillation With Diagram.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Explain Simple Distillation With Diagram Simple distillation is what you would use to get, for example, pure water from sea water. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in this ks3 chemistry guide from. Distillation separates a soluble substance from its. Explain Simple Distillation With Diagram.
From www.bbc.co.uk
Distillation BBC Bitesize Explain Simple Distillation With Diagram Distillation requires multiple pieces of. Distillation separates a soluble substance from its solvent based on differences in boiling points. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from. If you boil sea water,. Simple distillation is what you would use to get, for example, pure water from sea water. Simple distillation is. Explain Simple Distillation With Diagram.
From www.pinterest.co.uk
Distillation Process Science HowThingsWork Gif Distillation Explain Simple Distillation With Diagram If you boil sea water,. There is only one liquid present. Distillation separates a soluble substance from its solvent based on differences in boiling points. Distillation requires multiple pieces of. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry guide from. There are different ways to separate mixtures, such as. Explain Simple Distillation With Diagram.
From www.youtube.com
How To Draw Simple Distillation Diagram step by step YouTube Explain Simple Distillation With Diagram If you boil sea water,. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. There is only one liquid present. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation separates a soluble substance from its solvent based on differences in boiling points. Learn more in this ks3 chemistry. Explain Simple Distillation With Diagram.
From www.dreamstime.com
Simple Distillation Apparatus Diagram with Full Process Vector Explain Simple Distillation With Diagram If you boil sea water,. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation requires multiple pieces of. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Learn more in this ks3 chemistry guide. Explain Simple Distillation With Diagram.
From classnotes.org.in
Evaporation, Distillation Class 6, Separation of Substances Explain Simple Distillation With Diagram If you boil sea water,. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation requires multiple pieces of. There is only one liquid present. Simple distillation is what you would use to get, for example, pure water from sea water. Learn more in this ks3 chemistry guide from. Explain the role of the lever. Explain Simple Distillation With Diagram.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Explain Simple Distillation With Diagram There is only one liquid present. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Simple distillation is what you would use to get, for example, pure water from sea water. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation is a separation technique used to remove a solvent. Explain Simple Distillation With Diagram.
From www.yaclass.in
Simple distillation — lesson. Science CBSE, Class 9. Explain Simple Distillation With Diagram Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation separates a soluble substance from its solvent based on differences in boiling points. Simple distillation is what you would use to get, for example, pure water from sea water. Learn more in this ks3 chemistry guide from. There is only one liquid present. If you. Explain Simple Distillation With Diagram.
From www.pinterest.com
Fractional Distillation Vector Illustration Labeled Educational Stock Explain Simple Distillation With Diagram Distillation requires multiple pieces of. Simple distillation is a separation technique that capitalizes on differences in boiling points to. If you boil sea water,. Learn more in this ks3 chemistry guide from. There is only one liquid present. Explain the role of the lever rule in fractional. Distillation is a separation technique used to remove a solvent from a mixture. Explain Simple Distillation With Diagram.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy Explain Simple Distillation With Diagram Distillation requires multiple pieces of. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. There is only one liquid present. Distillation separates a soluble substance from its solvent based on differences in boiling points. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from. Simple distillation is a. Explain Simple Distillation With Diagram.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Explain Simple Distillation With Diagram Simple distillation is what you would use to get, for example, pure water from sea water. Learn more in this ks3 chemistry guide from. Distillation is a separation technique used to remove a solvent from a mixture and keep it. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. If you boil sea water,. Explain. Explain Simple Distillation With Diagram.
From mavink.com
Labelled Diagram Of Distillation Explain Simple Distillation With Diagram There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Learn more in this ks3 chemistry guide from. Distillation requires multiple pieces of. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Simple distillation is what you would use to get, for example, pure water from sea water. Distillation is a. Explain Simple Distillation With Diagram.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Explain Simple Distillation With Diagram There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Learn more in. Explain Simple Distillation With Diagram.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Explain Simple Distillation With Diagram There is only one liquid present. Simple distillation is a separation technique that capitalizes on differences in boiling points to. If you boil sea water,. Distillation requires multiple pieces of. Explain the role of the lever rule in fractional. Learn more in this ks3 chemistry guide from. Distillation is a separation technique used to remove a solvent from a mixture. Explain Simple Distillation With Diagram.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Explain Simple Distillation With Diagram Simple distillation is a separation technique that capitalizes on differences in boiling points to. There is only one liquid present. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Simple distillation is what you would use to get, for example,. Explain Simple Distillation With Diagram.
From www.youtube.com
Fractional distillation diagram / science diagram / important science Explain Simple Distillation With Diagram Distillation separates a soluble substance from its solvent based on differences in boiling points. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. If you boil sea water,. Explain the role of the lever rule in fractional. Distillation is a separation technique. Explain Simple Distillation With Diagram.
From www.vecteezy.com
Diagram showing Distillation Separating Mixtures 2896364 Vector Art at Explain Simple Distillation With Diagram There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Explain the role of the lever rule in fractional. Distillation separates a soluble substance from its solvent based on differences in boiling points. There is only one liquid present. Simple. Explain Simple Distillation With Diagram.
From mavink.com
Distillation Phase Diagram Explain Simple Distillation With Diagram Simple distillation is a separation technique that capitalizes on differences in boiling points to. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Simple distillation is what you would use to get, for example, pure water from sea water. There is only one liquid present. There are different ways to separate mixtures, such. Explain Simple Distillation With Diagram.
From madhumithapandiaraja207chemejournal.blogspot.com
Madhumitha's Chemistry EJournal Teacher's Demonstration Simple Explain Simple Distillation With Diagram There are different ways to separate mixtures, for example by filtration, crystallisation, distillation. Distillation is a separation technique used to remove a solvent from a mixture and keep it. Distillation requires multiple pieces of. Simple distillation is what you would use to get, for example, pure water from sea water. If you boil sea water,. Simple distillation is a separation. Explain Simple Distillation With Diagram.