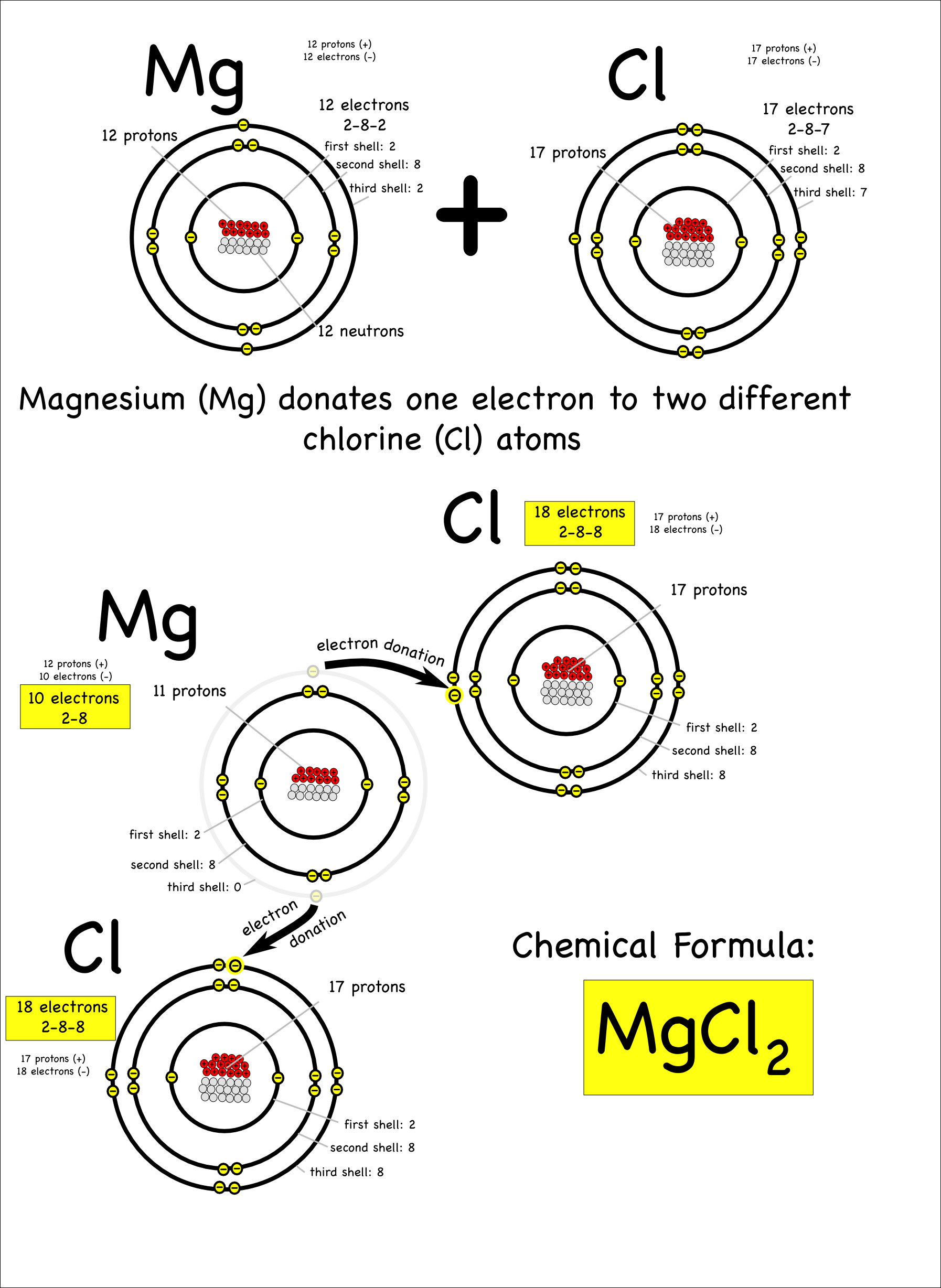
Mg Ionic Or Covalent . The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in.
from montessorimuddle.org
Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds generally form from metals and nonmetals. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Predict the type of compound formed from elements based on their location within the.
An Introduction to Ionic Bonding Montessori Muddle
Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Define ionic and molecular (covalent) compounds. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Ionic compounds generally form from metals and nonmetals. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one.
From www.pinterest.co.uk
Difference between ionic ,covalent and coordinate covalent bond Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Predict the type of compound formed from elements based on their location within the. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. A bond is. Mg Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Predict the type of compound formed from elements based on their location. Mg Ionic Or Covalent.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Mg Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds generally form from metals and nonmetals. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms. Mg Ionic Or Covalent.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. The properties described in chapter 6 the structure. Mg Ionic Or Covalent.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Mg Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly.. Mg Ionic Or Covalent.
From slideplayer.com
Types of Chemical Reactions ppt download Mg Ionic Or Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Predict the type of compound formed from elements based on their location within the. The simplist guide to the covalent or ionic character of a bond is. Mg Ionic Or Covalent.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Predict the type of. Mg Ionic Or Covalent.
From pediaa.com
Difference Between Covalent and Ionic Bonds Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends. Mg Ionic Or Covalent.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube Mg Ionic Or Covalent A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Predict the type of compound formed from elements based on their location within. Mg Ionic Or Covalent.
From techiescientist.com
Is MgO Ionic or Covalent? Techiescientist Mg Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. Ionic compounds generally form from metals and nonmetals. The properties described in chapter 6 the structure of atoms and chapter 7 the. Mg Ionic Or Covalent.
From slideplayer.com
Chapter 6 Chemical Reactions Occur in Predictable Ways ppt download Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Predict the. Mg Ionic Or Covalent.
From www.numerade.com
SOLVED classify the following compounds as ionic or covalent compounds Mg Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties. Mg Ionic Or Covalent.
From www.numerade.com
SOLVED 48. For each of the following compounds, state whether it is Mg Ionic Or Covalent A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds generally form from metals and. Mg Ionic Or Covalent.
From www.slideserve.com
PPT Introductory Chemistry , 2 nd Edition Nivaldo Tro PowerPoint Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Compounds that do not contain ions, but instead consist of atoms. Mg Ionic Or Covalent.
From clarence-well-duffy.blogspot.com
Explain the Major Differences Between Covalent and Ionic Bonding Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The simplist guide to the. Mg Ionic Or Covalent.
From www.slideserve.com
PPT Introductory Chemistry , 2 nd Edition Nivaldo Tro PowerPoint Mg Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. Ionic compounds generally form from metals and nonmetals. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The main difference between ionic and covalent bonds is how equally. Mg Ionic Or Covalent.
From topblogtenz.com
Is MgO ionic or covalent? Nature of chemical bond in MgO Mg Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The properties described in chapter 6 the structure of atoms and chapter. Mg Ionic Or Covalent.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Mg Ionic Or Covalent The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but. Mg Ionic Or Covalent.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Compounds that do not contain ions, but instead consist of atoms bonded tightly. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom. Mg Ionic Or Covalent.
From www.youtube.com
Lewis Structure of Magnesium Oxide (MgO) YouTube Mg Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Predict the type of compound formed from elements based on their location within the. The main difference between ionic and covalent bonds is how equally. Mg Ionic Or Covalent.
From www.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. Predict the type of compound formed from elements based. Mg Ionic Or Covalent.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how. Mg Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. The simplist guide to the covalent or ionic character of a bond is to consider the types of. Mg Ionic Or Covalent.
From catlnewtlynch.blogspot.com
Mgcl2 Ionic or Covalent CatlnewtLynch Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. Predict the type of compound formed from elements based on their location within. Mg Ionic Or Covalent.
From sciencenotes.org
Ionic vs Covalent Bonds Mg Ionic Or Covalent The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Ionic compounds generally form from metals and nonmetals.. Mg Ionic Or Covalent.
From www.numerade.com
SOLVED Classify these bonds as ionic, polar covalent, or nonpolar Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The simplist guide to the covalent or ionic character of a bond. Mg Ionic Or Covalent.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Mg Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Predict the type of compound formed from elements based on their location within the. The properties described in chapter 6 the. Mg Ionic Or Covalent.
From www.youtube.com
Is MgF2 (Magnesium fluoride) Ionic or Covalent? YouTube Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in. The main difference between ionic and covalent bonds is how equally the electrons. Mg Ionic Or Covalent.
From www.youtube.com
MgO Ionic or Covalent? Check the full Video YouTube Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. The simplist guide to the. Mg Ionic Or Covalent.
From www.slideshare.net
Covalent and ionic review do this first!!! Mg Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within. Mg Ionic Or Covalent.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Mg Ionic Or Covalent A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Compounds that do not contain ions, but instead consist of atoms. Mg Ionic Or Covalent.
From whatsinsight.org
Is MgCl2 Ionic or Covalent? What's Insight Mg Ionic Or Covalent Define ionic and molecular (covalent) compounds. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. The main difference between ionic and. Mg Ionic Or Covalent.
From topblogtenz.com
Is MgCl2 ionic or covalent? Nature of chemical bond in MgCl2 Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in.. Mg Ionic Or Covalent.
From case-has-robbins.blogspot.com
Which Best Compares the Properties of Ionic and Metallic Substances Mg Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent). Mg Ionic Or Covalent.
From www.numerade.com
SOLVED Text 2 out of 3 atoms Be sure to answer all parts Classify Mg Ionic Or Covalent The properties described in chapter 6 the structure of atoms and chapter 7 the periodic table and periodic trends were properties of. Predict the type of compound formed from elements based on their location within the. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from. Mg Ionic Or Covalent.