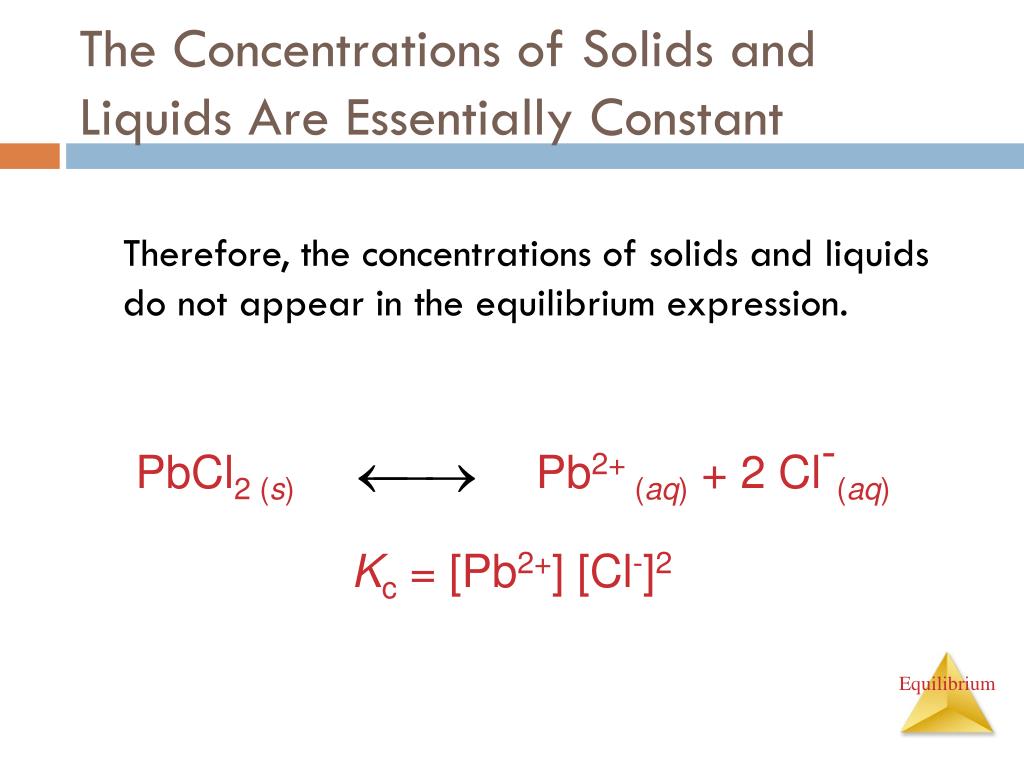
Solid-Liquid Equilibrium Examples . Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Take water (h 2 o) as an. Using a phase diagram (on p. 772 in chemistry3) the normal melting and. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Since a solid is very hard to transport over a process, we usually melt it into liquid form.
from www.slideserve.com
In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. 772 in chemistry3) the normal melting and. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Since a solid is very hard to transport over a process, we usually melt it into liquid form. Using a phase diagram (on p. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an.
PPT The Concept of Equilibrium PowerPoint Presentation, free download
Solid-Liquid Equilibrium Examples Take water (h 2 o) as an. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Since a solid is very hard to transport over a process, we usually melt it into liquid form. Take water (h 2 o) as an. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. 772 in chemistry3) the normal melting and. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Using a phase diagram (on p.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation ID3889861 Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. Using a phase diagram (on p. Since a solid is very hard to transport over a process, we usually melt it into liquid form. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. First, at a substance’s melting point or boiling point, two. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Solid-Liquid Equilibrium Examples First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: 772 in chemistry3) the normal melting and. Take water (h 2 o) as an. A state where both solid and liquid phases coexist in equilibrium at. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Lecture 17 Open Systems (Solid/Liquid Equilibrium) PowerPoint Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. Take water (h 2 o) as an. Using a phase diagram (on p. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: 772 in chemistry3) the normal melting and. A state. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT The Concept of Equilibrium PowerPoint Presentation, free download Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Since a solid is very hard to transport over a process, we usually melt it into liquid form. 772 in chemistry3) the normal melting and. A state where both solid and liquid phases coexist in equilibrium at. Solid-Liquid Equilibrium Examples.
From learncheme.com
ssleequilibrium LearnChemE Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Using a phase diagram (on. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Using a phase diagram (on p. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. Since a solid is very hard to transport over a process, we usually. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Equilibrium Basic Concepts PowerPoint Presentation, free download Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Take water (h 2 o) as an. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: First, at a substance’s melting point or. Solid-Liquid Equilibrium Examples.
From www.researchgate.net
Simple Binary SolidLiquid Equilibrium Diagram Download Scientific Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. 772 in chemistry3) the normal melting and. Take water (h 2 o) as an. Using a phase. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Unit 2 Liquids & solids, solubility, equilibrium PowerPoint Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Take water (h 2 o) as an. In. Solid-Liquid Equilibrium Examples.
From slideplayer.com
Introduction to the Equilibrium Constant ppt download Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. Take water (h 2 o) as an. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Consider an. Solid-Liquid Equilibrium Examples.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. Using a phase diagram (on p. Take water (h 2 o) as an. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Consider an equilibrium between a crystalline salt (or other kind. Solid-Liquid Equilibrium Examples.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. 772 in chemistry3) the normal melting and. Using a phase diagram (on p. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Consider an equilibrium between a. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Unit 2 Liquids and solids, solubility, equilibrium PowerPoint Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Using a phase diagram (on p. Consider an equilibrium between a crystalline salt (or other kind of ionic. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Solid-Liquid Equilibrium Examples A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. 772 in chemistry3) the normal melting and. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Take water (h 2 o) as an. In contrast, a system whose reactants, products,. Solid-Liquid Equilibrium Examples.
From slideplayer.com
Phase Changes. ppt download Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Using a phase diagram (on p. Since a solid is very hard to transport over a process, we usually melt it into liquid form. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a. Solid-Liquid Equilibrium Examples.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Using a phase diagram (on p. Take water (h 2 o) as an. Since a solid is very hard to transport over a process, we usually melt it into liquid. Solid-Liquid Equilibrium Examples.
From slideplayer.com
Chemical Equilibrium Lesson 1 Chapter ppt download Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Using a phase diagram (on p. Since a solid is very hard to transport over a process, we usually melt it into liquid form. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a. Solid-Liquid Equilibrium Examples.
From www.youtube.com
15.5 Heterogeneous Equilibria Reactions Involving a Solid or a Liquid Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. A state where. Solid-Liquid Equilibrium Examples.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Using a phase diagram (on p. In contrast,. Solid-Liquid Equilibrium Examples.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Solid-Liquid Equilibrium Examples First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Take water (h. Solid-Liquid Equilibrium Examples.
From slideplayer.com
Lesson 2 Equilibrium Law & Constants ppt download Solid-Liquid Equilibrium Examples Take water (h 2 o) as an. Using a phase diagram (on p. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Since a solid is very hard to transport over a process, we usually melt it into liquid form. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a. Solid-Liquid Equilibrium Examples.
From fyoxfhpqg.blob.core.windows.net
Solids And Liquids In Equilibrium Expressions at Royal Bessette blog Solid-Liquid Equilibrium Examples Take water (h 2 o) as an. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: First, at a substance’s melting point or boiling point, two phases can exist simultaneously.. Solid-Liquid Equilibrium Examples.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Using a phase diagram (on p. 772 in chemistry3) the normal melting and. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Since a solid is very hard to transport over a process, we usually. Solid-Liquid Equilibrium Examples.
From www.toppr.com
Equilibrium in Physical Processes SolidLiquidGas Equilibrium, Examples Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. 772 in chemistry3) the normal melting and. In contrast, a system whose reactants, products, or both are in more than one. Solid-Liquid Equilibrium Examples.
From studycorgi.com
SolidLiquid Equilibrium in a Binary System Free Essay Example Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Using a phase diagram (on p. First, at a substance’s melting point or boiling point, two phases can. Solid-Liquid Equilibrium Examples.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Since a solid is very hard to transport over a process, we usually melt it into liquid form. 772 in chemistry3) the normal. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500701 Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. 772 in chemistry3) the normal melting and. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In contrast, a system whose reactants, products, or both are in more than one phase. Solid-Liquid Equilibrium Examples.
From www.youtube.com
⚗️ Writing Equilibrium Expressions for Reactions Involving a Solid or a Solid-Liquid Equilibrium Examples Take water (h 2 o) as an. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: First, at a substance’s melting point or boiling point, two phases can exist simultaneously. 772 in chemistry3) the normal melting and. Using a phase diagram (on p. A state where both solid and. Solid-Liquid Equilibrium Examples.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid-Liquid Equilibrium Examples First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. 772 in chemistry3) the normal melting and. Since a solid is very hard to transport over a process, we usually melt it into liquid form. In contrast, a system whose reactants, products, or both are in more than one. Solid-Liquid Equilibrium Examples.
From www.slideserve.com
PPT Unit 2 Liquids and solids, solubility, equilibrium PowerPoint Solid-Liquid Equilibrium Examples Since a solid is very hard to transport over a process, we usually melt it into liquid form. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. Take water (h 2 o) as an. 772 in chemistry3) the normal melting and. First, at a substance’s melting point or boiling point,. Solid-Liquid Equilibrium Examples.
From www.snexplores.org
Explainer What are the different states of matter? Solid-Liquid Equilibrium Examples Using a phase diagram (on p. Take water (h 2 o) as an. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In contrast, a system whose reactants, products, or both are in more than. Solid-Liquid Equilibrium Examples.
From www.youtube.com
SolidLiquid Equilibrium YouTube Solid-Liquid Equilibrium Examples In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Using a phase diagram (on p. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Take water (h 2 o) as an. First, at a substance’s. Solid-Liquid Equilibrium Examples.
From www.numerade.com
SOLVED\( \mathrm{} \) \( \mathrm{} \) Solid-Liquid Equilibrium Examples A state where both solid and liquid phases coexist in equilibrium at a specific temperature and pressure, critical for. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. 772 in chemistry3) the normal melting and. Using a phase diagram (on p. First, at a substance’s melting. Solid-Liquid Equilibrium Examples.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Solid-Liquid Equilibrium Examples 772 in chemistry3) the normal melting and. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Using a phase diagram (on p. Since a solid is. Solid-Liquid Equilibrium Examples.
From ivypanda.com
SolidLiquid Equilibrium in Binary System Test 1922 Words Essay Example Solid-Liquid Equilibrium Examples Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Since a solid is very hard to transport over a process, we usually melt it into liquid form. In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the. Solid-Liquid Equilibrium Examples.