Topic 3.13 Beer-Lambert Law Answer Key . Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. There is a high probability that you will be assessed in beer. Every chemical species absorbs light at a given wavelength; Measure the absorption of a series of solutions at various known concentrations. Graph showing a calibration curve absorbance vs concentration. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Run a blank to account for solvent absorption. (a) the longest wavelength of light with enough. Study with quizlet and memorize flashcards containing terms like. The relationship between absorbance and concentration is _? Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Chemists use the absorbing properties of chemical compounds for quantitative.
from slidetodoc.com
Chemists use the absorbing properties of chemical compounds for quantitative. Measure the absorption of a series of solutions at various known concentrations. (a) the longest wavelength of light with enough. Study with quizlet and memorize flashcards containing terms like. Run a blank to account for solvent absorption. Every chemical species absorbs light at a given wavelength; Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. Graph showing a calibration curve absorbance vs concentration. There is a high probability that you will be assessed in beer. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100.
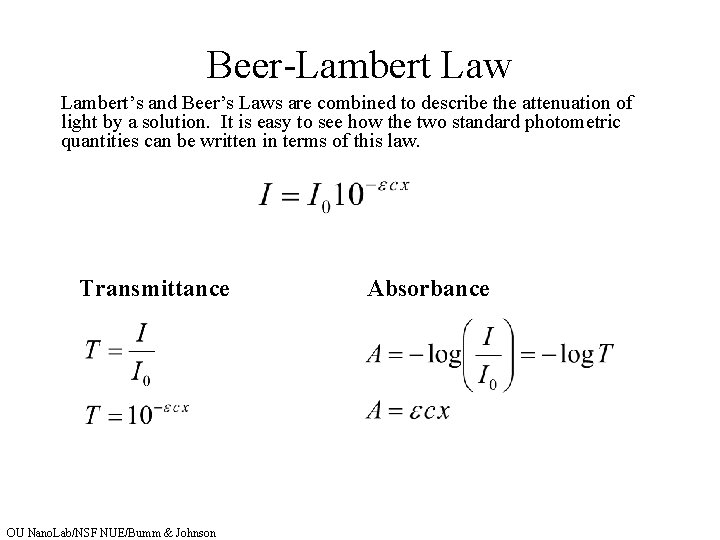
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers
Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. Every chemical species absorbs light at a given wavelength; Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Graph showing a calibration curve absorbance vs concentration. Run a blank to account for solvent absorption. (a) the longest wavelength of light with enough. The relationship between absorbance and concentration is _? Study with quizlet and memorize flashcards containing terms like. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Measure the absorption of a series of solutions at various known concentrations. There is a high probability that you will be assessed in beer. Chemists use the absorbing properties of chemical compounds for quantitative.
From study.com
How to Find the Path Length Using the BeerLambert Law Chemistry Topic 3.13 Beer-Lambert Law Answer Key There is a high probability that you will be assessed in beer. Chemists use the absorbing properties of chemical compounds for quantitative. Measure the absorption of a series of solutions at various known concentrations. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00. Topic 3.13 Beer-Lambert Law Answer Key.
From www.slideshare.net
Beer Lambert Law solved problems Topic 3.13 Beer-Lambert Law Answer Key There is a high probability that you will be assessed in beer. (a) the longest wavelength of light with enough. Graph showing a calibration curve absorbance vs concentration. Run a blank to account for solvent absorption. Chemists use the absorbing properties of chemical compounds for quantitative. The relationship between absorbance and concentration is _? Beer’s law is most effective with. Topic 3.13 Beer-Lambert Law Answer Key.
From www.youtube.com
3.13. BeerLambert Law College Board AP Chemistry YouTube Topic 3.13 Beer-Lambert Law Answer Key There is a high probability that you will be assessed in beer. Measure the absorption of a series of solutions at various known concentrations. The relationship between absorbance and concentration is _? F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to. Topic 3.13 Beer-Lambert Law Answer Key.
From www.slideshare.net
Introduction to beer lambert law Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. Measure the absorption of a series of solutions at various known concentrations. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Graph showing a calibration curve. Topic 3.13 Beer-Lambert Law Answer Key.
From chemistrypubs.com
BeerLambert Law Equation, Derivation, & Uses Chemistrupubs Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. Graph showing a calibration curve absorbance vs concentration. There is a high probability that you will be assessed in beer. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles. Topic 3.13 Beer-Lambert Law Answer Key.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Topic 3.13 Beer-Lambert Law Answer Key Measure the absorption of a series of solutions at various known concentrations. Graph showing a calibration curve absorbance vs concentration. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a. Topic 3.13 Beer-Lambert Law Answer Key.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. Measure the absorption of a series of solutions at various known concentrations. Study with quizlet and memorize flashcards containing terms like. Every chemical species absorbs light at a given wavelength; Chemists use the absorbing properties of chemical compounds for quantitative. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2. Topic 3.13 Beer-Lambert Law Answer Key.
From www.scribd.com
Beer Lambert Law Absorbance Mole (Unit) Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Study with quizlet and memorize flashcards containing terms like. Every chemical species absorbs light at a given wavelength;. Topic 3.13 Beer-Lambert Law Answer Key.
From www.chegg.com
Solved The BeerLambert Law is useful for determining the Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. There is a high probability that you will be assessed in beer. (a) the longest wavelength of light with enough. Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. The relationship between absorbance and concentration. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studypool.com
SOLUTION Beer lambert law Studypool Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. Every chemical species absorbs light at a given wavelength; The relationship between absorbance and concentration is _? Graph showing a calibration curve absorbance vs concentration. Chemists use the absorbing properties of chemical compounds for quantitative. Beer’s law is most effective with solutions that visibly change color over the course of a. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studypool.com
SOLUTION Beer lambert law, definition, history, derivation, diagram Topic 3.13 Beer-Lambert Law Answer Key Measure the absorption of a series of solutions at various known concentrations. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. (a) the longest wavelength of light with. Topic 3.13 Beer-Lambert Law Answer Key.
From www.scribd.com
Beer Lambert Law Problems Detailed Model Answer PDF PDF Absorbance Topic 3.13 Beer-Lambert Law Answer Key The relationship between absorbance and concentration is _? Every chemical species absorbs light at a given wavelength; Run a blank to account for solvent absorption. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3. Topic 3.13 Beer-Lambert Law Answer Key.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Topic 3.13 Beer-Lambert Law Answer Key Answer the following questions regarding light and its interactions with molecules, atoms, and ions. The relationship between absorbance and concentration is _? Run a blank to account for solvent absorption. Chemists use the absorbing properties of chemical compounds for quantitative. Every chemical species absorbs light at a given wavelength; Graph showing a calibration curve absorbance vs concentration. (a) the longest. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studocu.com
THE BEER Lambert LAW THE BEER LAMBERT LAW The BeerLambert law Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. Every chemical species absorbs light at a given wavelength; F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Beer’s law. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studocu.com
3.13 BeerLambert Law Practice Problems 3 BeerLambert Law Practice Topic 3.13 Beer-Lambert Law Answer Key Measure the absorption of a series of solutions at various known concentrations. There is a high probability that you will be assessed in beer. Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. Graph showing a calibration curve absorbance vs concentration. Chemists use the. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studypool.com
SOLUTION Beer Lambert Law Question and Answers Studypool Topic 3.13 Beer-Lambert Law Answer Key Run a blank to account for solvent absorption. Chemists use the absorbing properties of chemical compounds for quantitative. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Answer. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studypool.com
SOLUTION Beer Lambert Law Question and Answers Studypool Topic 3.13 Beer-Lambert Law Answer Key Measure the absorption of a series of solutions at various known concentrations. Graph showing a calibration curve absorbance vs concentration. Study with quizlet and memorize flashcards containing terms like. There is a high probability that you will be assessed in beer. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Every chemical species absorbs light. Topic 3.13 Beer-Lambert Law Answer Key.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Topic 3.13 Beer-Lambert Law Answer Key Measure the absorption of a series of solutions at various known concentrations. There is a high probability that you will be assessed in beer. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. F e. Topic 3.13 Beer-Lambert Law Answer Key.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Topic 3.13 Beer-Lambert Law Answer Key There is a high probability that you will be assessed in beer. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Study with quizlet and memorize flashcards containing terms like. Every chemical species absorbs light. Topic 3.13 Beer-Lambert Law Answer Key.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Topic 3.13 Beer-Lambert Law Answer Key F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Graph showing a calibration curve absorbance vs concentration. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) +. Topic 3.13 Beer-Lambert Law Answer Key.
From www.comsol.it
Modeling LaserMaterial Interactions with the BeerLambert Law COMSOL Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. Chemists use the absorbing properties of chemical compounds for quantitative. There is a high probability that you will be assessed in beer. (a) the longest wavelength of light with enough. Measure the absorption of a series of solutions at various known concentrations. F e 3 + a q + k s c. Topic 3.13 Beer-Lambert Law Answer Key.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. (a) the longest wavelength of light with enough. Measure the absorption of a series of solutions at various known concentrations. Chemists use the absorbing properties of chemical compounds for quantitative. F e 3 + a q + k s c n (s) → f e s c n 2 + (a. Topic 3.13 Beer-Lambert Law Answer Key.
From www.slideshare.net
Beer lamberts law PPT Topic 3.13 Beer-Lambert Law Answer Key (a) the longest wavelength of light with enough. Graph showing a calibration curve absorbance vs concentration. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. The relationship between. Topic 3.13 Beer-Lambert Law Answer Key.
From www.youtube.com
Spectrophotometry and the BeerLambert Law AP Chem Unit 3, Topic 13 Topic 3.13 Beer-Lambert Law Answer Key The relationship between absorbance and concentration is _? (a) the longest wavelength of light with enough. Run a blank to account for solvent absorption. Study with quizlet and memorize flashcards containing terms like. Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. Cu (s). Topic 3.13 Beer-Lambert Law Answer Key.
From sciencenotes.org
Beer's Law Equation and Example Topic 3.13 Beer-Lambert Law Answer Key Every chemical species absorbs light at a given wavelength; Graph showing a calibration curve absorbance vs concentration. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. There is a high probability that you will be. Topic 3.13 Beer-Lambert Law Answer Key.
From 6m999.com
BeerLambert Law Statement, Formula, Equation & Derivation / Numerical Topic 3.13 Beer-Lambert Law Answer Key (a) the longest wavelength of light with enough. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Every chemical species absorbs light at a given wavelength; The relationship between absorbance and concentration is _? Run a blank to account for solvent absorption. F e 3 + a q + k s c n (s) →. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studocu.com
BeerLambert Law Lecture notes with annotations CHEM0020 Studocu Topic 3.13 Beer-Lambert Law Answer Key The relationship between absorbance and concentration is _? (a) the longest wavelength of light with enough. Graph showing a calibration curve absorbance vs concentration. There is a high probability that you will be assessed in beer. Measure the absorption of a series of solutions at various known concentrations. Answer the following questions regarding light and its interactions with molecules, atoms,. Topic 3.13 Beer-Lambert Law Answer Key.
From chemtutorialbyanjanagopinadh.blogspot.com
Beerlamberts law Topic 3.13 Beer-Lambert Law Answer Key Study with quizlet and memorize flashcards containing terms like. (a) the longest wavelength of light with enough. Chemists use the absorbing properties of chemical compounds for quantitative. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f. Topic 3.13 Beer-Lambert Law Answer Key.
From www.slideshare.net
Introduction to beer lambert law Topic 3.13 Beer-Lambert Law Answer Key Graph showing a calibration curve absorbance vs concentration. (a) the longest wavelength of light with enough. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o (l) each student in a class placed a 2.00 g sample of a mixture of cu and. Run a blank to account for solvent absorption. Beer’s law. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studocu.com
Biochemistry Few Questions and answers based on BeerLambert Law and Topic 3.13 Beer-Lambert Law Answer Key F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. (a) the longest wavelength of light with enough. Study with quizlet and memorize flashcards containing terms like. Chemists use. Topic 3.13 Beer-Lambert Law Answer Key.
From rethinkbiologynotes.com
Spectroscopy and BeerLambert's Law Rethink Biology Notes Topic 3.13 Beer-Lambert Law Answer Key Every chemical species absorbs light at a given wavelength; Run a blank to account for solvent absorption. Study with quizlet and memorize flashcards containing terms like. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e. Topic 3.13 Beer-Lambert Law Answer Key.
From www.iitianacademy.com
AP Chemistry 3.13 BeerLambert Law Exam Style questions with Answer MCQ Topic 3.13 Beer-Lambert Law Answer Key Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Study with quizlet and memorize flashcards containing terms like. F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a. Topic 3.13 Beer-Lambert Law Answer Key.
From www.studypool.com
SOLUTION 2 beer lambert law complete Studypool Topic 3.13 Beer-Lambert Law Answer Key Chemists use the absorbing properties of chemical compounds for quantitative. The relationship between absorbance and concentration is _? Beer’s law is most effective with solutions that visibly change color over the course of a reaction, but if a spectrophotometer that emits light in. Cu (s) + 4 hno3 (aq) → cu (no3)2 (aq) + 2 no2 (g) + 2 h2o. Topic 3.13 Beer-Lambert Law Answer Key.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Topic 3.13 Beer-Lambert Law Answer Key F e 3 + a q + k s c n (s) → f e s c n 2 + (a q) + k + (a q) to determine the moles of f e 3 + (a q) in a 100. Run a blank to account for solvent absorption. There is a high probability that you will be assessed in. Topic 3.13 Beer-Lambert Law Answer Key.
From testbook.com
Derivation of BeerLambert Law Statement, Formula, Graph, Uses Topic 3.13 Beer-Lambert Law Answer Key There is a high probability that you will be assessed in beer. Graph showing a calibration curve absorbance vs concentration. The relationship between absorbance and concentration is _? Run a blank to account for solvent absorption. Every chemical species absorbs light at a given wavelength; Beer’s law is most effective with solutions that visibly change color over the course of. Topic 3.13 Beer-Lambert Law Answer Key.