Equipment Validation Guidelines . This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Find out when, how and why. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices.
from rs-ness.com
Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Find out when, how and why. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical.
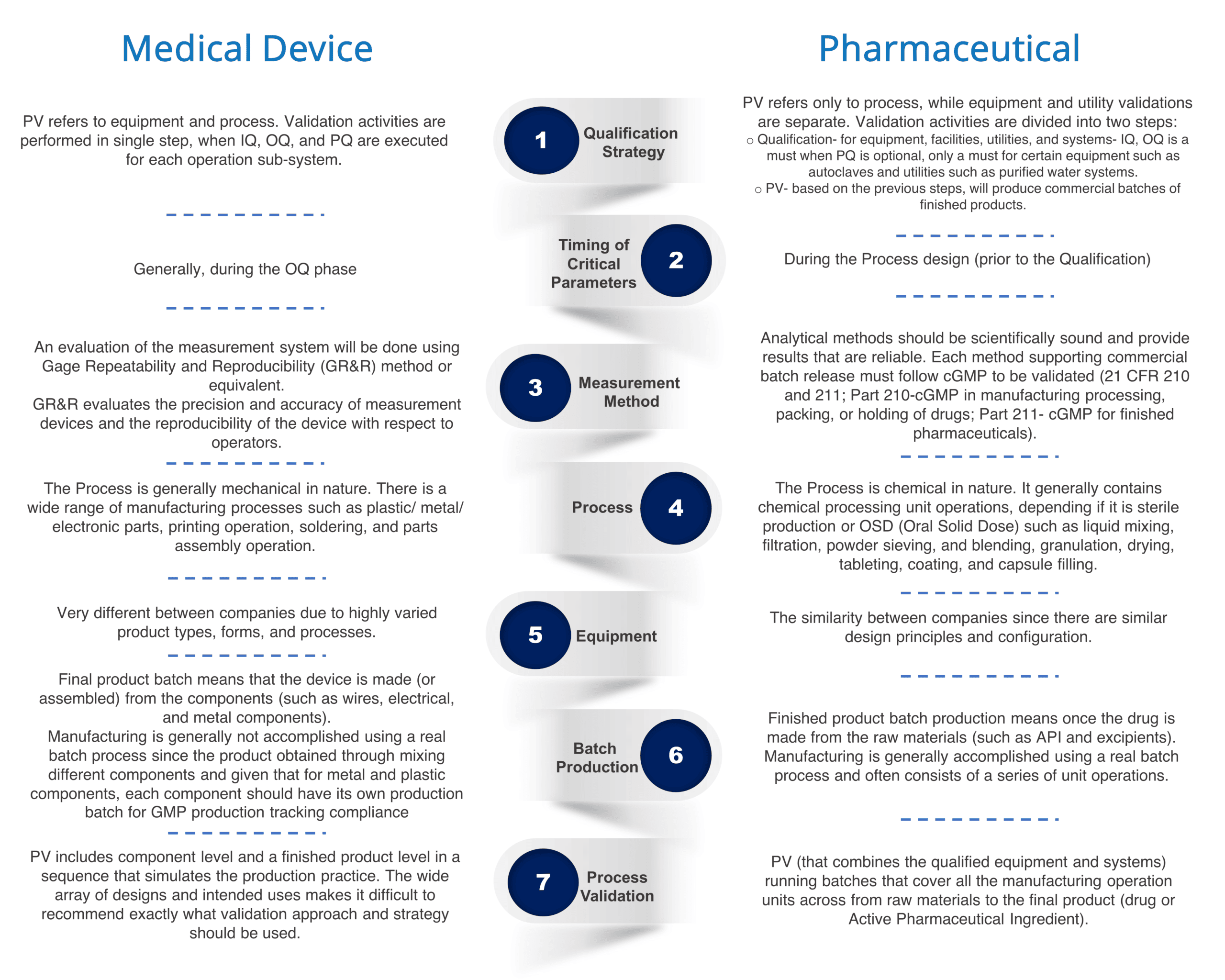
Process Validation Pharma vs. Medical Device RS NESS
Equipment Validation Guidelines This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Find out when, how and why. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical.
From www.researchgate.net
Process Validation, Equipment Selection and Qualification Download Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Find out when, how and why. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments. Equipment Validation Guidelines.
From www.researchgate.net
Process of equipment validation Download Scientific Diagram Equipment Validation Guidelines Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Find out when, how and why. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn. Equipment Validation Guidelines.
From www.orielstat.com
Medical Device Process Validation Plans Oriel STAT A MATRIX Equipment Validation Guidelines Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This document provides general. Equipment Validation Guidelines.
From www.slideshare.net
EQUIPMENT VALIDATION Equipment Validation Guidelines Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general principles. Equipment Validation Guidelines.
From www.slideserve.com
PPT Infrastructure Qualification (What Is a Network?) PowerPoint Equipment Validation Guidelines Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Find. Equipment Validation Guidelines.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Equipment Validation Guidelines Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Find out when, how and why. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn what iq, oq, pq. Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Equipment Validation Guidelines This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Equipment validation is a documented process demonstrating whether a. Equipment Validation Guidelines.
From pharmastate.academy
Operational Qualification (OQ) for Equipments Equipment Validation Guidelines Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Find out when,. Equipment Validation Guidelines.
From variation.com
Six Sigma Validation Process Taylor Enterprises Equipment Validation Guidelines Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Find out when, how and why. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including. Equipment Validation Guidelines.
From www.presentationeze.com
Equipment Validation FAT, DQ, IQ, OQ, PQ, URS.PresentationEZE Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Find out when, how and why. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within. Equipment Validation Guidelines.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. This document provides general principles and. Equipment Validation Guidelines.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn what iq, oq, pq are. Equipment Validation Guidelines.
From www.slideshare.net
Validation of equipment copy Equipment Validation Guidelines Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Find out when, how and why. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn about. Equipment Validation Guidelines.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Equipment Validation Guidelines Find out when, how and why. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This guidance. Equipment Validation Guidelines.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Equipment Validation Guidelines Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Find out when, how and why. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn what iq, oq,. Equipment Validation Guidelines.
From www.presentationeze.com
Equipment Validation PresentationEZE Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Equipment validation is a documented process demonstrating whether a. Equipment Validation Guidelines.
From www.micronhvac.com
Facility/Equipment Qualification & Validation Micron HVAC Pvt. Ltd Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn what iq, oq, pq. Equipment Validation Guidelines.
From www.kewaunee.in
HVAC System Validation Guidelines Kewaunee Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Find out when, how and why. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. This. Equipment Validation Guidelines.
From www.researchgate.net
Equipment qualification process. EQequipment qualification Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Find out when, how and why. Learn. Equipment Validation Guidelines.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Equipment Validation Guidelines Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. This document provides general principles and guidance. Equipment Validation Guidelines.
From es.scribd.com
15 Equipment Qualification PDF Equipment Validation Guidelines Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Find out when, how and why. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about. Equipment Validation Guidelines.
From studylib.net
VAL090EquipmentValidationGuidelinesample Equipment Validation Guidelines Find out when, how and why. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and. Equipment Validation Guidelines.
From www.slideshare.net
Equipment validation Equipment Validation Guidelines Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This document provides general. Equipment Validation Guidelines.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Equipment Validation Guidelines This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Find out when, how and why. Learn how laboratories can use iq/oq/pq. Equipment Validation Guidelines.
From www.complianceteamllc.com
How To Move, Qualify & Validate Your Manufacturing Facility And Equipment Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Find out when, how and why. Learn about the fda and ghtf requirements and recommendations for process validation of. Equipment Validation Guidelines.
From www.linkedin.com
COMPUTER SYSTEM VALIDATION Equipment Validation Guidelines Find out when, how and why. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Equipment validation is a documented process. Equipment Validation Guidelines.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Equipment Validation Guidelines Find out when, how and why. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices,. Equipment Validation Guidelines.
From www.getreskilled.com
Equipment Validation Protocol Step by Step Writing Guide Equipment Validation Guidelines This document provides general principles and guidance on validation of computerized systems for pharmaceutical production and control. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn about. Equipment Validation Guidelines.
From issuu.com
The Scope of Pharma Computer System Validation by Company Connect Equipment Validation Guidelines Find out when, how and why. Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and. Equipment Validation Guidelines.
From pharmdguru.com
4. GLP (Good Laboratory Practice), ISO (The International Organization Equipment Validation Guidelines This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Find out when, how and why. Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation.. Equipment Validation Guidelines.
From www.cmitest.nl
Equipment Validation HVAC Cleanroom Management International Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. This guidance outlines the. Equipment Validation Guidelines.
From www.pdffiller.com
Fillable Online Equipment Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. This guidance outlines. Equipment Validation Guidelines.
From www.cmitest.nl
Equipment Validation HVAC Cleanroom Management International Equipment Validation Guidelines Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical. Equipment Validation Guidelines.
From www.getreskilled.com
Download 4 professional IQ OQ PQ templates GetReskilled Equipment Validation Guidelines Learn about the cgmp requirements for facilities and equipment, including aseptic processing, cleanrooms, and cleaning validation. This guidance outlines the general principles and approaches for process validation of drugs and biologics, including active pharmaceutical. Learn how laboratories can use iq/oq/pq (installation, operational, performance qualification) to verify and validate equipment or instruments used to make. Equipment validation is a documented process. Equipment Validation Guidelines.
From pharmaanalytic.com
Equipment Qualification PharmaAnalytic LLC GMP, Quality Management Equipment Validation Guidelines Learn about the fda and ghtf requirements and recommendations for process validation of medical devices. Learn what iq, oq, pq are and why they are important for equipment validation in pharmaceutical, medical devices, and clinical industries. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined. This guidance outlines the. Equipment Validation Guidelines.