Potassium Hydroxide And Iron Iii Sulfate Empirical Formula . Complete the table below by deciding whether a precipitate forms. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Calcium ions have a charge of 2+, while. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Enter an equation of an ionic chemical equation and press the balance button.
from clarktivesoto.blogspot.com
The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the balance button. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Complete the table below by deciding whether a precipitate forms. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. The balanced equation will be calculated along with the.
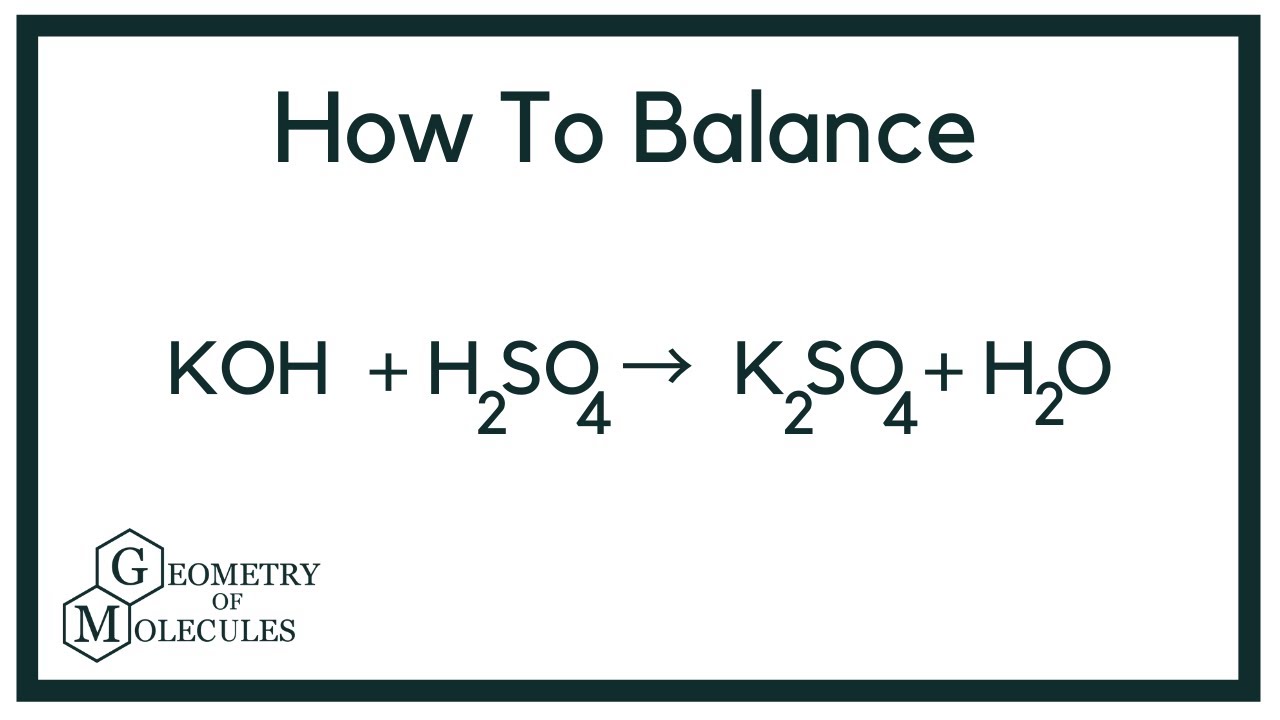
Potassium Hydroxide and Sulphuric Acid
Potassium Hydroxide And Iron Iii Sulfate Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Enter an equation of an ionic chemical equation and press the balance button. Calcium ions have a charge of 2+, while. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Complete the table below by deciding whether a precipitate forms.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Calcium ions have a charge of 2+, while. Complete the table below by deciding whether a precipitate forms. Enter an equation. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.chegg.com
Solved Complete the table below by deciding whether a Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Complete the table below by deciding whether a precipitate forms. The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Enter an equation of an ionic chemical equation and press the balance button. For. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. We will need two potassium ions to balance the charge on the sulfate ion, so the. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Calcium ions have a charge of. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.ibresco.com
Ammonium Iron (II) sulfate hexahydrate آمونیوم فروس سولفات 6 آبه Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation will be calculated along with the. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Enter an equation of an ionic chemical equation and press the balance button. Complete the table below by deciding whether a precipitate forms. Calcium ions have a charge of 2+, while. Write the. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From brainly.in
Write the balanced chemical equation for the following reaction Barium Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Write the balanced chemical equation for the reaction between iron (iii) sulfate. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.numerade.com
What mass of iron(III) hydroxide precipitate can be produced by Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: The balanced. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.youtube.com
Equation for Fe2(SO4)3 + H2O Iron (III) sulfate + Water YouTube Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.toppr.com
The formula of iron (III) sulphate is Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation will be calculated along with the. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds containing only monatomic ions (such as nacl) and for many compounds. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.toppr.com
Write the net ionic equation for the reaction of potassium dichromate Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Enter an equation of an ionic chemical equation and press the balance button. Calcium ions have a charge of 2+, while. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Complete. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From ar.inspiredpencil.com
Iron Iii Sulfate Atoms Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Complete the table below by deciding whether a precipitate forms. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Calcium ions have a charge of 2+, while. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\). Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From fr.thptnganamst.edu.vn
Ntroduire 95+ imagen sulfate de sodium formule fr.thptnganamst.edu.vn Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Write the balanced chemical. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Enter an equation of an ionic chemical equation and press the balance button. Complete the table below by deciding whether a precipitate forms. Calcium ions have a charge of 2+, while. For compounds containing only monatomic. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). For compounds containing only monatomic ions (such. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.bartleby.com
Answered Complete the table below by deciding… bartleby Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. The balanced equation will be calculated along with the. Complete the table below by deciding whether a precipitate forms. We will. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From brainly.in
Show me the formula of following salt by criss cross method 1 Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Complete the table below by deciding whether a precipitate forms. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Write the balanced chemical equation for the reaction between iron. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.youtube.com
Reaction between Magnesium Sulfate and Sodium Carbonate Precipitation Potassium Hydroxide And Iron Iii Sulfate Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Complete the table below by deciding whether a precipitate forms. The balanced equation will be calculated along with the. Calcium ions have. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.youtube.com
The Reaction Between Iron (III) Nitrate and Sodium Hydroxide YouTube Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. For compounds containing only monatomic ions (such as nacl) and for many. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.bartleby.com
Answered Complete the table below by deciding… bartleby Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the balance button. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and.. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.toppr.com
What happen whenPotassium ferrocyanide solution is added to a solution Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation will be calculated along with the. Complete the table below by deciding whether a precipitate forms. Calcium ions have a charge of 2+, while. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. We will need two potassium ions to balance the charge on the sulfate ion, so. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.youtube.com
How to Write the Formula for Iron (III) hydroxide YouTube Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds containing only monatomic ions (such as nacl) and for. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From clarktivesoto.blogspot.com
Potassium Hydroxide and Sulphuric Acid Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Calcium ions have a charge of 2+, while. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: The balanced equation will be calculated along with the. Complete the table below by deciding whether a precipitate forms. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The balanced equation. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Enter an equation of an ionic chemical equation and press the balance button. For compounds containing only monatomic ions (such as. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Enter an equation of an ionic chemical equation and press the balance button. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Calcium ions have a charge of 2+, while. Complete the table below by deciding whether a precipitate forms. We will need two potassium ions to balance the charge on the sulfate ion,. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From brainly.in
what do you observe when ammonium hydroxide is added to iron(II Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Calcium ions have a charge of 2+, while. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. For. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.numerade.com
SOLVED solution A solution B Does a precipitate form when A and B are Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Enter an equation of an ionic chemical equation and press the balance button. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Complete the table below by deciding whether a precipitate forms. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). The. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From ar.inspiredpencil.com
Potassium Hydroxide Structure Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the balance button. For compounds containing only monatomic ions (such as nacl). Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Complete the table below by deciding whether a precipitate forms. Calcium ions have a charge of 2+, while. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Iron(iii)sulfate. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Potassium Hydroxide And Iron Iii Sulfate Empirical Formula The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. The balanced equation will be calculated along with the. Calcium ions have a charge of 2+, while. Write the balanced chemical equation. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Enter an equation of an ionic chemical equation and press the balance button. Calcium ions have a charge of 2+, while. The balanced equation will be calculated along with the. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From bayviewhotelandapartments.com
potassium permanganate and iron sulfate equation Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for iron sulfate (feso4) reacting with sodium hydroxide (naoh) to form iron hydroxide (fe(oh)3) and. Calcium ions have a charge of 2+, while. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. The. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.youtube.com
ALEKS Predicting Precipitation YouTube Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Complete the table below by deciding whether a precipitate forms. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Calcium ions have a charge of 2+, while. Enter an equation of an ionic chemical equation and press the balance button.. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Write the balanced chemical equation for the reaction between iron. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.slideserve.com
PPT Precipitation Reactions (Double Replacement Reactions) PowerPoint Potassium Hydroxide And Iron Iii Sulfate Empirical Formula We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{k_2so_4}\). Enter an equation of an ionic chemical equation and press the balance button. Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: For compounds. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.
From www.chegg.com
Solved A) potassium sulfide C) iron(III) hydroxide B) Potassium Hydroxide And Iron Iii Sulfate Empirical Formula Write the balanced chemical equation for the reaction between iron (iii) sulfate \((\text{fe}_2(\text{so}_4)_3)\) and potassium. Complete the table below by deciding whether a precipitate forms. The balanced equation will be calculated along with the. Iron(iii)sulfate + potassiumhydroxide = potassiumsulfate + iron(iii)hydroxide reaction type: Enter an equation of an ionic chemical equation and press the balance button. Calcium ions have a. Potassium Hydroxide And Iron Iii Sulfate Empirical Formula.