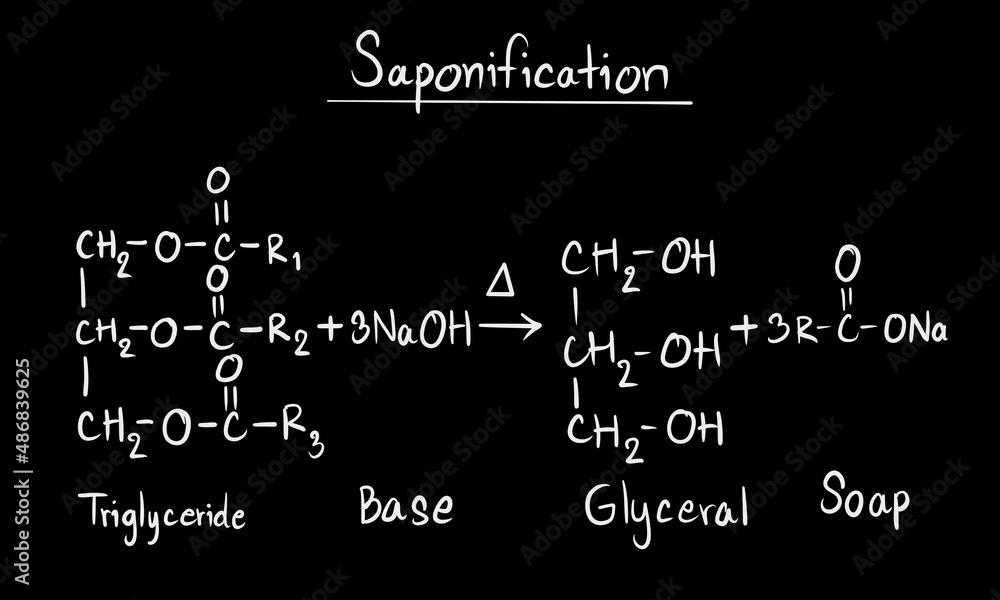
What Is The Chemistry Of Soap . Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Before sodium hydroxide was commercially available, a boiling solution of potassium. [ 1 ] in a domestic setting, soaps,. The reaction produces sodium salts of. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Each soap molecule has a long.
from stock.adobe.com
The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Each soap molecule has a long. The reaction produces sodium salts of. Before sodium hydroxide was commercially available, a boiling solution of potassium. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. [ 1 ] in a domestic setting, soaps,. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil.
Saponification equation, reaction of soap, chemistry equation of soap
What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is able to clean hands and dishes because of some pretty nifty chemistry. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Each soap molecule has a long. The reaction produces sodium salts of. Before sodium hydroxide was commercially available, a boiling solution of potassium. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. [ 1 ] in a domestic setting, soaps,.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The reaction produces sodium salts of. Soap is a salt of a fatty acid (sometimes other. What Is The Chemistry Of Soap.
From studylib.net
THE SCIENCE OF SOAPS AND DETERGENTS What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. [ 1 ] in a domestic setting, soaps,. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other. What Is The Chemistry Of Soap.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap What Is The Chemistry Of Soap Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water.. What Is The Chemistry Of Soap.
From richkosh.blogspot.com
EXAMS AND ME Soaps and Detergents What Is The Chemistry Of Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. How soap works is due to its unique chemistry, the hydrophilic (loves water). What Is The Chemistry Of Soap.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences What Is The Chemistry Of Soap Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap is a salt of. What Is The Chemistry Of Soap.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project What Is The Chemistry Of Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The reaction produces sodium salts of. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. [ 1 ] in a domestic setting, soaps,. Each soap molecule has a long. Before sodium hydroxide. What Is The Chemistry Of Soap.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry What Is The Chemistry Of Soap Each soap molecule has a long. Before sodium hydroxide was commercially available, a boiling solution of potassium. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. How soap works is due to its unique chemistry, the. What Is The Chemistry Of Soap.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab What Is The Chemistry Of Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able to clean hands and dishes because of some pretty nifty chemistry. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Before sodium hydroxide was commercially available, a boiling solution of potassium. The reaction. What Is The Chemistry Of Soap.
From www.scribd.com
Soap Soap Chemical Substances What Is The Chemistry Of Soap Before sodium hydroxide was commercially available, a boiling solution of potassium. The reaction produces sodium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. [ 1 ] in a domestic setting, soaps,. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Each soap molecule has a long. Soaps are. What Is The Chemistry Of Soap.
From www.pinterest.co.uk
Hand washing with soap vector illustration. Educational explanation What Is The Chemistry Of Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act. What Is The Chemistry Of Soap.
From www.walmart.com
The Chemistry of Soaps and Salts Chemistry Book for Beginners What Is The Chemistry Of Soap Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. The reaction produces sodium salts of. Before sodium hydroxide was commercially available, a boiling solution of potassium. Each soap molecule has a long. Soap molecules have on one end what’s known as a polar salt, which is. What Is The Chemistry Of Soap.
From cosmosmagazine.com
The chemistry of soap What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. Before sodium hydroxide was commercially available, a boiling solution of potassium. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease,. What Is The Chemistry Of Soap.
From www.thoughtco.com
How Soap Works What Is The Chemistry Of Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Each soap molecule has a long. Soap molecules have on one end what’s known as a polar salt, which. What Is The Chemistry Of Soap.
From www.reddit.com
Chemistry of Soap versus Body Wash r/chemistry What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or. What Is The Chemistry Of Soap.
From www.goodreads.com
Soap Chemistry Discover The Basics Of Soap Chemistry by Wan Yamane What Is The Chemistry Of Soap Each soap molecule has a long. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. The reaction produces sodium salts of. How soap works is due to. What Is The Chemistry Of Soap.
From steemit.com
Saponification 1/2 — Steemit What Is The Chemistry Of Soap Before sodium hydroxide was commercially available, a boiling solution of potassium. [ 1 ] in a domestic setting, soaps,. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act. What Is The Chemistry Of Soap.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. [ 1 ] in. What Is The Chemistry Of Soap.
From aliceinchemiland.blogspot.my
Organic Chemistry in My Daily Life Organic Chemistry about Soap and What Is The Chemistry Of Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The reaction produces sodium salts of. [ 1 ] in a domestic setting, soaps,. Soaps are cleaning agents that are usually made by reacting alkali (e.g.,. What Is The Chemistry Of Soap.
From brainly.in
what is the difference between the molecules of soap and detergents What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. [ 1 ] in a domestic setting, soaps,. The chemistry of soap and detergents unveils the science that underlies our. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. [ 1 ] in a domestic setting, soaps,. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other. What Is The Chemistry Of Soap.
From www.youtube.com
The Chemistry of Soap YouTube What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. The reaction produces sodium salts of. The chemistry of soap. What Is The Chemistry Of Soap.
From www.youtube.com
Chemistry 101 How does soap work? YouTube What Is The Chemistry Of Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able to clean hands and dishes because of some pretty nifty chemistry. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease,. What Is The Chemistry Of Soap.
From www.youtube.com
Types of Soap, Chemistry Lecture Sabaq.pk YouTube What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. Each soap molecule has a long. Before sodium hydroxide was commercially available, a boiling solution of potassium. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The reaction produces sodium salts of. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap. What Is The Chemistry Of Soap.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 What Is The Chemistry Of Soap Before sodium hydroxide was commercially available, a boiling solution of potassium. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. [ 1 ] in a domestic setting, soaps,. Soap is able to. What Is The Chemistry Of Soap.
From stock.adobe.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK What Is The Chemistry Of Soap Before sodium hydroxide was commercially available, a boiling solution of potassium. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Each soap molecule has a long. The reaction produces sodium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able. What Is The Chemistry Of Soap.
From sukachem.blogspot.com
Suka Chemistry SPM Form 5 Chemicals for Consumers (Checklist) What Is The Chemistry Of Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap The reaction produces sodium salts of. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able to. What Is The Chemistry Of Soap.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog What Is The Chemistry Of Soap Before sodium hydroxide was commercially available, a boiling solution of potassium. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able to clean hands and dishes because of some pretty nifty chemistry. [ 1 ] in a domestic setting, soaps,. Soaps are cleaning agents that are usually. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap [ 1 ] in a domestic setting, soaps,. The reaction produces sodium salts of. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. Each soap molecule has a long. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted. What Is The Chemistry Of Soap.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more What Is The Chemistry Of Soap Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. The reaction produces sodium salts of. Soap is able. What Is The Chemistry Of Soap.
From www.slideshare.net
Chemistry of soaps What Is The Chemistry Of Soap Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap molecule has a long. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids.. What Is The Chemistry Of Soap.
From www.youtube.com
Cleansing action of soap Chemical reactions Chemistry YouTube What Is The Chemistry Of Soap How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. Before sodium hydroxide was commercially available, a. What Is The Chemistry Of Soap.