What Temp Does Water Boil At In A Vacuum . What is the boiling point of water at low pressure ? If you want to see this for yourself, here’s an experiment you. 95 rows water boiling point at vacuum pressure. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. However, when pressure decreases, as in a. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. You would need a small. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and. The boiling point of a substance is the temperature at which the. When water is heated up under vacuum, its boiling point is lower than at atmospheric. This is why if you go to a high altitude location (like many parts of. Water actually boils at a lower temperature if the pressure around it is lowered.
from vacaero.com
However, when pressure decreases, as in a. Water actually boils at a lower temperature if the pressure around it is lowered. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. What is the boiling point of water at low pressure ? You would need a small. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. If you want to see this for yourself, here’s an experiment you. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a substance is the temperature at which the.
Simple Physics for the High Vacuum Processing Industry
What Temp Does Water Boil At In A Vacuum If you want to see this for yourself, here’s an experiment you. When water is heated up under vacuum, its boiling point is lower than at atmospheric. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and. However, when pressure decreases, as in a. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. If you want to see this for yourself, here’s an experiment you. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. 95 rows water boiling point at vacuum pressure. Water actually boils at a lower temperature if the pressure around it is lowered. You would need a small. What is the boiling point of water at low pressure ? This is why if you go to a high altitude location (like many parts of. The boiling point of a substance is the temperature at which the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful What Temp Does Water Boil At In A Vacuum However, when pressure decreases, as in a. 95 rows water boiling point at vacuum pressure. This is why if you go to a high altitude location (like many parts of. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a substance is the temperature at which the.. What Temp Does Water Boil At In A Vacuum.
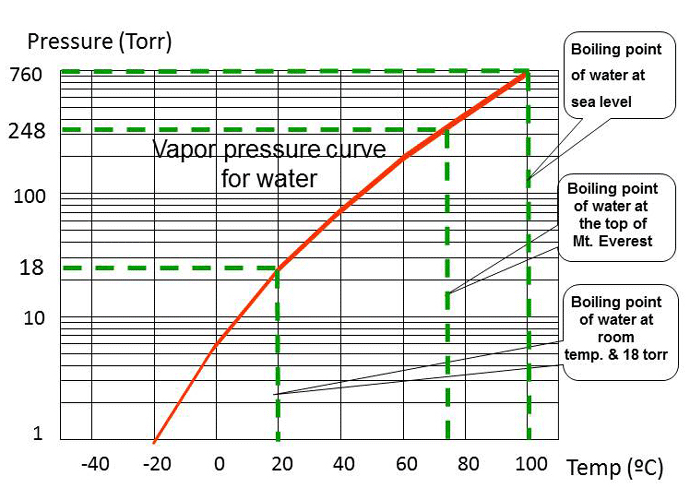
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts What Temp Does Water Boil At In A Vacuum The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a substance is the temperature at which the. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. This is why if you go to a high altitude location (like. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Does Water Really Boil in a Vacuum Chamber? And Why? YouTube What Temp Does Water Boil At In A Vacuum When water is heated up under vacuum, its boiling point is lower than at atmospheric. This is why if you go to a high altitude location (like many parts of. 95 rows water boiling point at vacuum pressure. Water actually boils at a lower temperature if the pressure around it is lowered. Online calculator, figures and tables giving the boiling. What Temp Does Water Boil At In A Vacuum.
From klajghnsh.blob.core.windows.net
What Is The Temperature For Boiling Water at Paul Makin blog What Temp Does Water Boil At In A Vacuum If you want to see this for yourself, here’s an experiment you. This is why if you go to a high altitude location (like many parts of. The boiling point of a substance is the temperature at which the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. In fact, you. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Boiling cold water In a Vacuum Chamber YouTube What Temp Does Water Boil At In A Vacuum This is why if you go to a high altitude location (like many parts of. However, when pressure decreases, as in a. The boiling point of a substance is the temperature at which the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The water will begin boiling and will hold. What Temp Does Water Boil At In A Vacuum.
From cleaningbeasts.com
What Temperature Does Water Boil In A Vacuum Cleaning Beasts What Temp Does Water Boil At In A Vacuum Water actually boils at a lower temperature if the pressure around it is lowered. What is the boiling point of water at low pressure ? Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. This is why if you go to a high altitude location (like many parts of. However, when. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Why does water boil in a vacuum? YouTube What Temp Does Water Boil At In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. When water is heated up under vacuum, its boiling point is lower than at atmospheric. However, when pressure decreases, as in a. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure. What Temp Does Water Boil At In A Vacuum.
From cegwohic.blob.core.windows.net
Why Does Water Boil In A Vacuum at Mary Riding blog What Temp Does Water Boil At In A Vacuum You would need a small. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. What is the boiling point of water at low pressure ? However, when pressure decreases, as in a. Water actually boils at a lower temperature if the pressure around it is lowered. This is why if you go to a high altitude location. What Temp Does Water Boil At In A Vacuum.
From chartdata.web.app
Water Boiling Point Pressure Chart What Temp Does Water Boil At In A Vacuum The boiling point of a substance is the temperature at which the. 95 rows water boiling point at vacuum pressure. If you want to see this for yourself, here’s an experiment you. However, when pressure decreases, as in a. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. What is the. What Temp Does Water Boil At In A Vacuum.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Temp Does Water Boil At In A Vacuum The boiling point of a substance is the temperature at which the. This is why if you go to a high altitude location (like many parts of. When water is heated up under vacuum, its boiling point is lower than at atmospheric. If you want to see this for yourself, here’s an experiment you. Online calculator, figures and tables giving. What Temp Does Water Boil At In A Vacuum.
From cejtzhsb.blob.core.windows.net
What Is The Boiling Point Of Water Under Vacuum at Vicki Williams blog What Temp Does Water Boil At In A Vacuum Water actually boils at a lower temperature if the pressure around it is lowered. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The boiling point of a substance is the temperature at which the. What is the boiling point of water at low pressure ? The water will begin boiling. What Temp Does Water Boil At In A Vacuum.
From dxolovigi.blob.core.windows.net
Why Does Water Boil At A Lower Temperature In A Vacuum at Marcy Miller blog What Temp Does Water Boil At In A Vacuum The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units.. What Temp Does Water Boil At In A Vacuum.
From mavink.com
Water Boiling Point Pressure Chart What Temp Does Water Boil At In A Vacuum Water actually boils at a lower temperature if the pressure around it is lowered. You would need a small. However, when pressure decreases, as in a. What is the boiling point of water at low pressure ? This is why if you go to a high altitude location (like many parts of. The boiling point of a substance is the. What Temp Does Water Boil At In A Vacuum.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent What Temp Does Water Boil At In A Vacuum Water actually boils at a lower temperature if the pressure around it is lowered. You would need a small. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. What is the boiling point of water at low pressure ? The boiling point of water is the temperature at which the vapor. What Temp Does Water Boil At In A Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature What Temp Does Water Boil At In A Vacuum If you want to see this for yourself, here’s an experiment you. However, when pressure decreases, as in a. What is the boiling point of water at low pressure ? The boiling point of a substance is the temperature at which the. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases.. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Boil Water Room Temperature YouTube What Temp Does Water Boil At In A Vacuum The boiling point of a substance is the temperature at which the. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. This is why if you go to a high altitude location (like many parts of. Water actually boils at a lower temperature if the pressure around it is lowered. However, when pressure decreases, as in a.. What Temp Does Water Boil At In A Vacuum.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Temp Does Water Boil At In A Vacuum What is the boiling point of water at low pressure ? Water actually boils at a lower temperature if the pressure around it is lowered. If you want to see this for yourself, here’s an experiment you. You would need a small. When water is heated up under vacuum, its boiling point is lower than at atmospheric. Online calculator, figures. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Temp Does Water Boil At In A Vacuum For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. What is the boiling point of water at low pressure ? Water actually boils at a lower temperature if the pressure around it is lowered. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a. What Temp Does Water Boil At In A Vacuum.
From scindustrialsales.com
Boiling Point of Water Under Vacuum Pumps and Filtration What Temp Does Water Boil At In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. If you want to see this for yourself, here’s an experiment you.. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
boiling water in vacuum chamber YouTube What Temp Does Water Boil At In A Vacuum In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. What is the boiling point of water at low pressure ? You would need a small. When water is heated up under vacuum, its boiling point is lower than at atmospheric. The water will begin boiling and will hold the chamber at. What Temp Does Water Boil At In A Vacuum.
From www.neuronmagazine.com
What Temperature Does Water Boil At 101 What Temp Does Water Boil At In A Vacuum In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. When water is heated up under vacuum, its boiling point is lower than at atmospheric. However, when pressure decreases, as in a. What is the boiling point of water at low pressure ? For example, water typically boils at 100°c (212°f) at. What Temp Does Water Boil At In A Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry What Temp Does Water Boil At In A Vacuum However, when pressure decreases, as in a. If you want to see this for yourself, here’s an experiment you. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. 95 rows water boiling point at vacuum pressure. What is the boiling point of water at low pressure ? The water will begin. What Temp Does Water Boil At In A Vacuum.
From www.acs.org
What Temperature Does Water Boil At? American Chemical Society What Temp Does Water Boil At In A Vacuum This is why if you go to a high altitude location (like many parts of. When water is heated up under vacuum, its boiling point is lower than at atmospheric. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. 95 rows water boiling point at vacuum pressure. The water will begin. What Temp Does Water Boil At In A Vacuum.
From blog.sciencescore.com
What temperature does water boil? What Temp Does Water Boil At In A Vacuum 95 rows water boiling point at vacuum pressure. You would need a small. This is why if you go to a high altitude location (like many parts of. The boiling point of a substance is the temperature at which the. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The boiling. What Temp Does Water Boil At In A Vacuum.
From cegwohic.blob.core.windows.net
Why Does Water Boil In A Vacuum at Mary Riding blog What Temp Does Water Boil At In A Vacuum You would need a small. The boiling point of a substance is the temperature at which the. Water actually boils at a lower temperature if the pressure around it is lowered. However, when pressure decreases, as in a. This is why if you go to a high altitude location (like many parts of. The water will begin boiling and will. What Temp Does Water Boil At In A Vacuum.
From mr.kentchemistry.com
Determine Boiling Point from Vapor Pressure What Temp Does Water Boil At In A Vacuum The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a substance is the temperature at which the. You would need a small. This is why if you go to a high altitude location (like many parts of. In fact, you can actually get a liquid to boil. What Temp Does Water Boil At In A Vacuum.
From www.cuemath.com
Celsius to Fahrenheit Conversion Formula Examples What Temp Does Water Boil At In A Vacuum What is the boiling point of water at low pressure ? In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. This is why if you go to a high altitude location (like many. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
What Temperature does Water Boil? YouTube What Temp Does Water Boil At In A Vacuum In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The boiling point of a substance is the temperature at which the. You would need a small. What is the boiling point of water at low pressure ? If you want to see this for yourself, here’s an experiment you. Water actually. What Temp Does Water Boil At In A Vacuum.
From dxolovigi.blob.core.windows.net
Why Does Water Boil At A Lower Temperature In A Vacuum at Marcy Miller blog What Temp Does Water Boil At In A Vacuum The boiling point of a substance is the temperature at which the. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. If you want to see this for yourself, here’s an experiment you. However, when pressure decreases, as in a. 95 rows water boiling point at vacuum pressure. Online calculator, figures. What Temp Does Water Boil At In A Vacuum.
From dxolovigi.blob.core.windows.net
Why Does Water Boil At A Lower Temperature In A Vacuum at Marcy Miller blog What Temp Does Water Boil At In A Vacuum 95 rows water boiling point at vacuum pressure. When water is heated up under vacuum, its boiling point is lower than at atmospheric. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. Water. What Temp Does Water Boil At In A Vacuum.
From www.youtube.com
Vacuum pump boiling water at room temperature YouTube What Temp Does Water Boil At In A Vacuum This is why if you go to a high altitude location (like many parts of. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. The boiling point of a substance is the temperature at which the. 95 rows water boiling point at vacuum pressure. The boiling point of water is the. What Temp Does Water Boil At In A Vacuum.
From www.myengineeringtools.com
Water boiling point vs pressure and vacuum What Temp Does Water Boil At In A Vacuum You would need a small. However, when pressure decreases, as in a. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. When water is heated up under vacuum, its boiling point is lower than at atmospheric. 95 rows water boiling point at vacuum pressure. The boiling point of a substance is. What Temp Does Water Boil At In A Vacuum.
From dxolovigi.blob.core.windows.net
Why Does Water Boil At A Lower Temperature In A Vacuum at Marcy Miller blog What Temp Does Water Boil At In A Vacuum If you want to see this for yourself, here’s an experiment you. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. For example, water typically boils at 100°c (212°f) at standard atmospheric pressure. Water actually boils at a lower temperature if the pressure around it is lowered. Online calculator, figures and. What Temp Does Water Boil At In A Vacuum.
From physics.stackexchange.com
thermodynamics If I boil water at room temp using a vacuum, will the What Temp Does Water Boil At In A Vacuum Water actually boils at a lower temperature if the pressure around it is lowered. 95 rows water boiling point at vacuum pressure. In fact, you can actually get a liquid to boil at room temperature if you have a vacuum. The boiling point of a substance is the temperature at which the. The water will begin boiling and will hold. What Temp Does Water Boil At In A Vacuum.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Temp Does Water Boil At In A Vacuum The boiling point of a substance is the temperature at which the. The water will begin boiling and will hold the chamber at an elevated pressure until that process ceases. However, when pressure decreases, as in a. When water is heated up under vacuum, its boiling point is lower than at atmospheric. If you want to see this for yourself,. What Temp Does Water Boil At In A Vacuum.