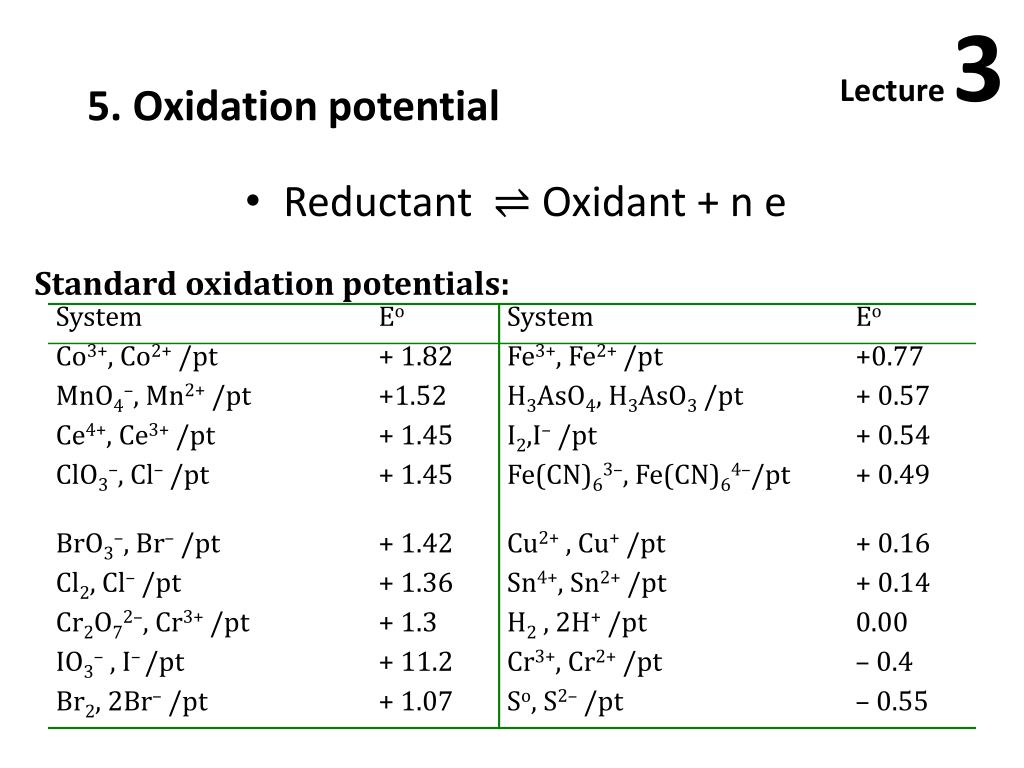
Standard Reduction Potential Strongest Oxidizing Agent . Find out which element is the strongest. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn how to use the standard reduction potential table to compare and predict redox reactions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. See a table of standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Find out which substances are oxidizing agents, reducing agents, and their. Identifying trends in oxidizing and reducing. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials.
from www.slideserve.com
How to use a table of standard reduction potentials to calculate standard cell potential. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Learn how to use the standard reduction potential table to compare and predict redox reactions. Identifying trends in oxidizing and reducing. See a table of standard potentials. Find out which substances are oxidizing agents, reducing agents, and their. Find out which element is the strongest. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials.
PPT OxidationReduction Reactions PowerPoint Presentation, free
Standard Reduction Potential Strongest Oxidizing Agent Find out which element is the strongest. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Identifying trends in oxidizing and reducing. Learn how to use the standard reduction potential table to compare and predict redox reactions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Find out which element is the strongest. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. How to use a table of standard reduction potentials to calculate standard cell potential. Find out which substances are oxidizing agents, reducing agents, and their. See a table of standard potentials. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials.
From www.vedantu.com
Oxidising Agent Definition, Factors Affecting It and Applications for JEE Standard Reduction Potential Strongest Oxidizing Agent Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Identifying trends in oxidizing and reducing. Find out which. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Given the standard reduction potentials Ce3+ + e > Ce2+ (+1. Standard Reduction Potential Strongest Oxidizing Agent Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. How to use a table of standard reduction potentials to calculate standard cell potential. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Learn how to compare the oxidative and reductive. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Using the table of standard electrode potentials, which of the Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. See a table of standard potentials. Learn how to use the standard reduction potential table to compare and predict redox reactions. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. See a table of standard potentials. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Learn how to use the standard reduction potential table to compare and predict redox reactions. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard. Standard Reduction Potential Strongest Oxidizing Agent.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Strongest Oxidizing Agent A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. See a table of standard potentials. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Identifying trends in oxidizing and reducing. Find out which substances are oxidizing. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Question 6 (2 points) Using the Standard Reduction Potential Standard Reduction Potential Strongest Oxidizing Agent See a table of standard potentials. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Find out which substances are oxidizing agents, reducing agents, and their. Learn how to. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Using the table of standard reduction potentials provided Standard Reduction Potential Strongest Oxidizing Agent A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Find out which element is the strongest. See a table of standard potentials. Learn how to use the standard reduction potential table to compare and predict redox reactions. Learn how to compare the oxidative and reductive strengths of various. Standard Reduction Potential Strongest Oxidizing Agent.
From www.chegg.com
Solved Which is the strongest oxidizing agent? Standard Standard Reduction Potential Strongest Oxidizing Agent How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to use the standard reduction potential table to compare and predict redox reactions. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn about oxidizing agents, their roles in oxidation and reduction. Standard Reduction Potential Strongest Oxidizing Agent.
From www.toppr.com
The standard reduction potentials at 25^∘C of Li^+/Li, Ba^2 + /Ba, Na Standard Reduction Potential Strongest Oxidizing Agent Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Identifying trends in oxidizing and reducing. How to use. Standard Reduction Potential Strongest Oxidizing Agent.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Standard Reduction Potential Strongest Oxidizing Agent Find out which substances are oxidizing agents, reducing agents, and their. Learn how to use the standard reduction potential table to compare and predict redox reactions. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several. Standard Reduction Potential Strongest Oxidizing Agent.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Strongest Oxidizing Agent Find out which substances are oxidizing agents, reducing agents, and their. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Learn how to use standard reduction potentials to predict redox reactions,. Standard Reduction Potential Strongest Oxidizing Agent.
From slidetodoc.com
Standard Reduction Potential The potential generated by a Standard Reduction Potential Strongest Oxidizing Agent See a table of standard potentials. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn how to use the standard reduction potential table to compare and predict redox reactions. Find out which. Standard Reduction Potential Strongest Oxidizing Agent.
From www.youtube.com
Aleks Ranking the strength of oxidizing and reducing agents using Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Use standard reduction potentials. Standard Reduction Potential Strongest Oxidizing Agent.
From www.chem.fsu.edu
Lab Purpose Standard Reduction Potential Strongest Oxidizing Agent Learn how to use the standard reduction potential table to compare and predict redox reactions. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT Oxidizing & Reducing Agents PowerPoint Presentation, free Standard Reduction Potential Strongest Oxidizing Agent Find out which substances are oxidizing agents, reducing agents, and their. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Find out which element is the strongest. Identifying trends in oxidizing and reducing. Use standard reduction potentials to determine the. Standard Reduction Potential Strongest Oxidizing Agent.
From slideplayer.com
Unit 12 Electrochemistry ppt download Standard Reduction Potential Strongest Oxidizing Agent Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. How to use a table of standard reduction potentials to calculate standard cell potential. See a table of standard potentials. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Identifying trends in oxidizing. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Based on the halfreactions and their respective standard Standard Reduction Potential Strongest Oxidizing Agent Learn how to use the standard reduction potential table to compare and predict redox reactions. How to use a table of standard reduction potentials to calculate standard cell potential. See a table of standard potentials. Find out which substances are oxidizing agents, reducing agents, and their. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Drag and drop the substances given below to rank them in Standard Reduction Potential Strongest Oxidizing Agent Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Identifying trends in oxidizing and reducing. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. See a table of standard potentials. How. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Which one of the below species will be the strongest reducing Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. See a table of standard potentials. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Learn how. Standard Reduction Potential Strongest Oxidizing Agent.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. How to use a table of standard reduction potentials to calculate standard cell potential. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Find out which element is the. Standard Reduction Potential Strongest Oxidizing Agent.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Strongest Oxidizing Agent See a table of standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Find out which. Standard Reduction Potential Strongest Oxidizing Agent.
From loensgcfn.blob.core.windows.net
Standard Reduction Potential Calculator at James Burkley blog Standard Reduction Potential Strongest Oxidizing Agent Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. See a table of standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based. Standard Reduction Potential Strongest Oxidizing Agent.
From solvedlib.com
Using the table of standard reduction potentials, whi… SolvedLib Standard Reduction Potential Strongest Oxidizing Agent Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn how to use the standard reduction potential table to compare and predict redox reactions. Use standard reduction potentials to determine the better oxidizing. Standard Reduction Potential Strongest Oxidizing Agent.
From schoolbag.info
Why is a strong oxidizing agent easy to reduce? Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. See a table of standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVEDUse the table of standard reduction potentials below to identify Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Find out which substances are oxidizing agents, reducing agents, and their. Use standard reduction potentials to determine the. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT Electrochemical Cells (aka Galvanic or Voltaic Cells Standard Reduction Potential Strongest Oxidizing Agent Find out which element is the strongest. See a table of standard potentials. Learn how to use the standard reduction potential table to compare and predict redox reactions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Find out which substances are oxidizing agents, reducing agents, and their. Learn how to use. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6601383 Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. Learn how to use the standard reduction potential table to compare and predict redox reactions. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. How to use a table of standard reduction potentials to calculate standard cell potential. Learn how to predict. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 20 PowerPoint Presentation, free Standard Reduction Potential Strongest Oxidizing Agent Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Find out which substances are oxidizing agents, reducing agents, and their. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Identifying trends in oxidizing and reducing. Find out which element is the strongest.. Standard Reduction Potential Strongest Oxidizing Agent.
From www.chegg.com
Solved OxidationReduction Table Strongest Oxidizing Agent Standard Reduction Potential Strongest Oxidizing Agent A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Find out which element is the strongest. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Find out which substances are oxidizing agents, reducing agents, and their. Use standard. Standard Reduction Potential Strongest Oxidizing Agent.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Standard Reduction Potential Strongest Oxidizing Agent A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. See a table of standard potentials. How. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED By consideration of the appropriate standard reduction Standard Reduction Potential Strongest Oxidizing Agent A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Find out which substances are oxidizing agents, reducing agents, and their. Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. See a table of standard potentials. Use. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Which of the following is the strongest oxidizing agent among Standard Reduction Potential Strongest Oxidizing Agent Learn how to use standard reduction potentials to predict redox reactions, calculate equilibrium constants, and identify powerful oxidizing and reducing agents. Learn how to compare the oxidative and reductive strengths of various substances using standard potentials. See a table of standard potentials. Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Using the data from Table 3, list the halfcell reactions in Standard Reduction Potential Strongest Oxidizing Agent Identifying trends in oxidizing and reducing. How to use a table of standard reduction potentials to calculate standard cell potential. Find out which element is the strongest. See a table of standard potentials. A pdf document with a table of standard reduction potentials for various elements, ions and compounds, relative to the standard hydrogen. Learn how to predict the relative. Standard Reduction Potential Strongest Oxidizing Agent.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Standard Reduction Potential Strongest Oxidizing Agent Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. Identifying trends in oxidizing and reducing. Learn how to use the standard reduction potential table to compare and predict redox reactions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Learn how to. Standard Reduction Potential Strongest Oxidizing Agent.
From www.numerade.com
SOLVED Ln (be) Question 10 (1 point) Using the provided table of Standard Reduction Potential Strongest Oxidizing Agent Learn about oxidizing agents, their roles in oxidation and reduction reactions, and their relative strengths based on standard electrode. See a table of standard potentials. Identifying trends in oxidizing and reducing. Learn how to predict the relative strengths of various oxidants and reductants using standard potentials. Find out which element is the strongest. How to use a table of standard. Standard Reduction Potential Strongest Oxidizing Agent.