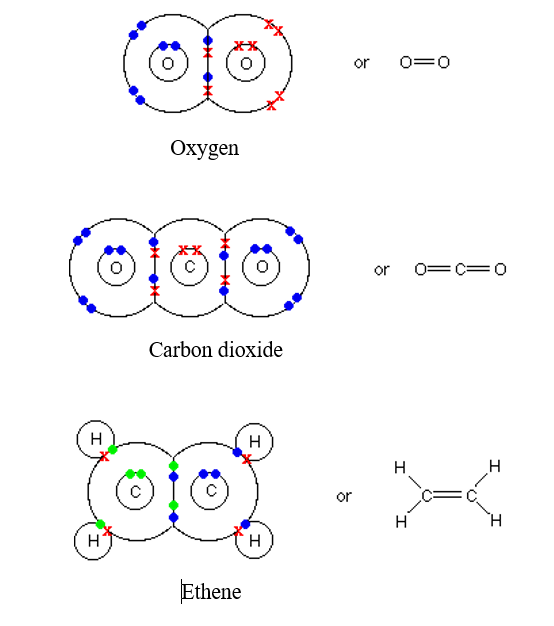
How Many Types Of Bonds Can Carbon Atom Form . In a single bond, two carbon atoms share one pair of. carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. carbon can form single, double, or even triple bonds with other carbon atoms. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Well, carbon can form up to four covalent bonds. With hydrogen, nitrogen, oxygen, and other. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. A carbon atom can form the following bonds: — the three major types of covalent bonds are single, double, and triple bonds.
from chemdictionary.org
A carbon atom can form the following bonds: With hydrogen, nitrogen, oxygen, and other. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Well, carbon can form up to four covalent bonds. In a single bond, two carbon atoms share one pair of. carbon can form single, double, or even triple bonds with other carbon atoms. Therefore, it can form four covalent bonds with other atoms or molecules. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. — the three major types of covalent bonds are single, double, and triple bonds. carbon contains four electrons in its outer shell.
Double Covalent Bond Facts, Definition, History & Examples
How Many Types Of Bonds Can Carbon Atom Form carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. With hydrogen, nitrogen, oxygen, and other. A carbon atom can form the following bonds: carbon can form single, double, or even triple bonds with other carbon atoms. In a single bond, two carbon atoms share one pair of. — the three major types of covalent bonds are single, double, and triple bonds. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Well, carbon can form up to four covalent bonds. carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules.
From www.pinterest.com
Four covalent bonds. Carbon has four valence electrons and here a How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. With hydrogen, nitrogen, oxygen, and other. carbon can form single, double, or even triple bonds with other carbon atoms. Well, carbon can form up to four covalent bonds. In a single bond, two carbon atoms share one pair of. carbon contains four electrons. How Many Types Of Bonds Can Carbon Atom Form.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Well, carbon can form up to four covalent bonds. Therefore, it can form four covalent bonds with other atoms or molecules.. How Many Types Of Bonds Can Carbon Atom Form.
From dxomztejs.blob.core.windows.net
What Are The Three Types Of Bonding In Chemistry at Andrea Hoeppner blog How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. With hydrogen, nitrogen, oxygen, and other. In a single bond, two carbon atoms share one pair of. carbon can form single, double, or even triple bonds with other carbon atoms. A carbon atom can form the following bonds: as carbon atoms are added to a molecular framework, the carbon. How Many Types Of Bonds Can Carbon Atom Form.
From fabalabse.com
What are the 4 types bonds? Leia aqui What are the 4 types of bonds How Many Types Of Bonds Can Carbon Atom Form carbon can form single, double, or even triple bonds with other carbon atoms. Well, carbon can form up to four covalent bonds. A carbon atom can form the following bonds: With hydrogen, nitrogen, oxygen, and other. Therefore, it can form four covalent bonds with other atoms or molecules. — the three major types of covalent bonds are single,. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID89333 How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. Therefore, it can form four covalent bonds with other atoms or molecules. carbon can form single, double, or even triple bonds with other carbon atoms. With hydrogen, nitrogen, oxygen, and other. carbon contains four electrons in its outer shell. Well, carbon can form. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Carbon The backbone of life PowerPoint Presentation, free How Many Types Of Bonds Can Carbon Atom Form A carbon atom can form the following bonds: In a single bond, two carbon atoms share one pair of. Therefore, it can form four covalent bonds with other atoms or molecules. Well, carbon can form up to four covalent bonds. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures.. How Many Types Of Bonds Can Carbon Atom Form.
From www.sliderbase.com
Organic Chemistry Presentation Chemistry How Many Types Of Bonds Can Carbon Atom Form Well, carbon can form up to four covalent bonds. With hydrogen, nitrogen, oxygen, and other. carbon contains four electrons in its outer shell. In a single bond, two carbon atoms share one pair of. — the three major types of covalent bonds are single, double, and triple bonds. Therefore, it can form four covalent bonds with other atoms. How Many Types Of Bonds Can Carbon Atom Form.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy How Many Types Of Bonds Can Carbon Atom Form With hydrogen, nitrogen, oxygen, and other. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Therefore, it can form four covalent bonds with other atoms or molecules. carbon contains four electrons in its outer shell. In a single bond, two carbon atoms share one. How Many Types Of Bonds Can Carbon Atom Form.
From www.alamy.com
Carbon Atomic Structure High Resolution Stock Photography and Images How Many Types Of Bonds Can Carbon Atom Form Well, carbon can form up to four covalent bonds. Therefore, it can form four covalent bonds with other atoms or molecules. A carbon atom can form the following bonds: carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. carbon contains four electrons in its. How Many Types Of Bonds Can Carbon Atom Form.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds How Many Types Of Bonds Can Carbon Atom Form carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. With hydrogen, nitrogen, oxygen, and other. carbon contains four electrons in its outer shell. A. How Many Types Of Bonds Can Carbon Atom Form.
From www.macmillanhighered.com
hillis2e_ch02 How Many Types Of Bonds Can Carbon Atom Form carbon can form single, double, or even triple bonds with other carbon atoms. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. carbon. How Many Types Of Bonds Can Carbon Atom Form.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds How Many Types Of Bonds Can Carbon Atom Form Well, carbon can form up to four covalent bonds. With hydrogen, nitrogen, oxygen, and other. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon contains four electrons in its outer shell. — the three major types of covalent bonds are single, double, and triple bonds. . How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID89333 How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. — the three major types of covalent bonds are single, double, and triple bonds. A carbon atom can form the following bonds: as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon can form single, double, or even. How Many Types Of Bonds Can Carbon Atom Form.
From study.com
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript How Many Types Of Bonds Can Carbon Atom Form carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. Therefore, it can form four covalent bonds with other atoms or molecules. carbon can form single, double, or even triple bonds with other carbon atoms. — the three major types of covalent bonds are. How Many Types Of Bonds Can Carbon Atom Form.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology How Many Types Of Bonds Can Carbon Atom Form as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. carbon contains four electrons in its outer shell. A carbon atom can form the following. How Many Types Of Bonds Can Carbon Atom Form.
From sciencenotes.org
Carbon Compounds and Examples How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. With hydrogen, nitrogen, oxygen, and other. Therefore, it can form four covalent bonds with other atoms or molecules. In a single. How Many Types Of Bonds Can Carbon Atom Form.
From tydesnhsteele.blogspot.com
List and Explain the Five Different Types of Bonds How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. Well, carbon can form up to four covalent bonds. In a single bond, two carbon atoms share one pair of. With hydrogen, nitrogen, oxygen, and other. A carbon atom can form the following bonds: Therefore, it can form four covalent bonds with other atoms or. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID89333 How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. A carbon atom can form the following bonds: carbon can form single, double, or even triple bonds with other carbon atoms. In a. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. carbon can form single, double, or even triple bonds with other carbon atoms. With hydrogen, nitrogen, oxygen, and other. A carbon atom can form the following bonds: as carbon atoms are added to a molecular framework, the. How Many Types Of Bonds Can Carbon Atom Form.
From saylordotorg.github.io
Naming Covalent Compounds How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. With hydrogen, nitrogen, oxygen, and other. Well, carbon can form up to four covalent bonds. — the three major types of covalent bonds are single, double, and triple bonds. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron,. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Organic Chemistry Functional Groups PowerPoint Presentation How Many Types Of Bonds Can Carbon Atom Form A carbon atom can form the following bonds: as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. Well, carbon can form up to four covalent bonds. With hydrogen, nitrogen, oxygen, and other. carbon can form single, double, or even triple bonds with other carbon atoms. In a single. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Carbon Compounds PowerPoint Presentation, free download ID2319022 How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. In a single bond, two carbon atoms share one pair of. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon can form single, double, or even triple bonds with other carbon atoms.. How Many Types Of Bonds Can Carbon Atom Form.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy How Many Types Of Bonds Can Carbon Atom Form With hydrogen, nitrogen, oxygen, and other. carbon can form single, double, or even triple bonds with other carbon atoms. A carbon atom can form the following bonds: carbon contains four electrons in its outer shell. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. — the. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Carbon PowerPoint Presentation, free download ID1833140 How Many Types Of Bonds Can Carbon Atom Form — the three major types of covalent bonds are single, double, and triple bonds. In a single bond, two carbon atoms share one pair of. With hydrogen, nitrogen, oxygen, and other. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon, with four valence electrons, forms covalent. How Many Types Of Bonds Can Carbon Atom Form.
From slidetodoc.com
Chapter 8 Covalent Bonding Covalent bonding Usually forms How Many Types Of Bonds Can Carbon Atom Form Therefore, it can form four covalent bonds with other atoms or molecules. With hydrogen, nitrogen, oxygen, and other. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as. How Many Types Of Bonds Can Carbon Atom Form.
From quizlet.com
How many covalent bonds can a single carbon (C) atom form? Quizlet How Many Types Of Bonds Can Carbon Atom Form carbon can form single, double, or even triple bonds with other carbon atoms. With hydrogen, nitrogen, oxygen, and other. A carbon atom can form the following bonds: In a single bond, two carbon atoms share one pair of. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron,. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT Introduction to Carbon Chemistry PowerPoint Presentation, free How Many Types Of Bonds Can Carbon Atom Form carbon can form single, double, or even triple bonds with other carbon atoms. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. A carbon atom can form the following bonds: In a single bond, two carbon atoms share one pair of. as carbon. How Many Types Of Bonds Can Carbon Atom Form.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation, free download ID3105916 How Many Types Of Bonds Can Carbon Atom Form With hydrogen, nitrogen, oxygen, and other. Well, carbon can form up to four covalent bonds. as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. In a single bond, two carbon atoms share one pair of. carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms. How Many Types Of Bonds Can Carbon Atom Form.
From unacademy.com
Covalent Bonding in Carbon Atom How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. With hydrogen, nitrogen, oxygen, and other. In a single bond, two carbon atoms share one pair of. Well, carbon can form up to four covalent bonds. carbon can form single, double, or even triple bonds with other carbon. How Many Types Of Bonds Can Carbon Atom Form.
From edurev.in
Overview of Carbon and Covalent Bonding in Carbon Class 10 Notes EduRev How Many Types Of Bonds Can Carbon Atom Form In a single bond, two carbon atoms share one pair of. A carbon atom can form the following bonds: carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in. — the three major types of covalent bonds are single, double, and triple bonds. as. How Many Types Of Bonds Can Carbon Atom Form.
From chemdictionary.org
Double Covalent Bond Facts, Definition, History & Examples How Many Types Of Bonds Can Carbon Atom Form carbon contains four electrons in its outer shell. — the three major types of covalent bonds are single, double, and triple bonds. carbon can form single, double, or even triple bonds with other carbon atoms. Well, carbon can form up to four covalent bonds. Therefore, it can form four covalent bonds with other atoms or molecules. . How Many Types Of Bonds Can Carbon Atom Form.
From www.goldhilleducation.co.uk
Atomic structure Mychem How Many Types Of Bonds Can Carbon Atom Form Therefore, it can form four covalent bonds with other atoms or molecules. With hydrogen, nitrogen, oxygen, and other. Well, carbon can form up to four covalent bonds. A carbon atom can form the following bonds: as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon contains four electrons. How Many Types Of Bonds Can Carbon Atom Form.
From slideplayer.com
Chapter 4 Carbon Do Now How many bonds can carbon form? ppt download How Many Types Of Bonds Can Carbon Atom Form carbon can form single, double, or even triple bonds with other carbon atoms. Well, carbon can form up to four covalent bonds. carbon contains four electrons in its outer shell. — the three major types of covalent bonds are single, double, and triple bonds. Therefore, it can form four covalent bonds with other atoms or molecules. . How Many Types Of Bonds Can Carbon Atom Form.
From lauryn-has-curry.blogspot.com
What Is Stored in Carbon Bonds LaurynhasCurry How Many Types Of Bonds Can Carbon Atom Form A carbon atom can form the following bonds: With hydrogen, nitrogen, oxygen, and other. carbon can form single, double, or even triple bonds with other carbon atoms. — the three major types of covalent bonds are single, double, and triple bonds. carbon contains four electrons in its outer shell. as carbon atoms are added to a. How Many Types Of Bonds Can Carbon Atom Form.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table How Many Types Of Bonds Can Carbon Atom Form as carbon atoms are added to a molecular framework, the carbon chain can develop branches or form cyclic structures. carbon can form single, double, or even triple bonds with other carbon atoms. With hydrogen, nitrogen, oxygen, and other. A carbon atom can form the following bonds: carbon contains four electrons in its outer shell. carbon, with. How Many Types Of Bonds Can Carbon Atom Form.