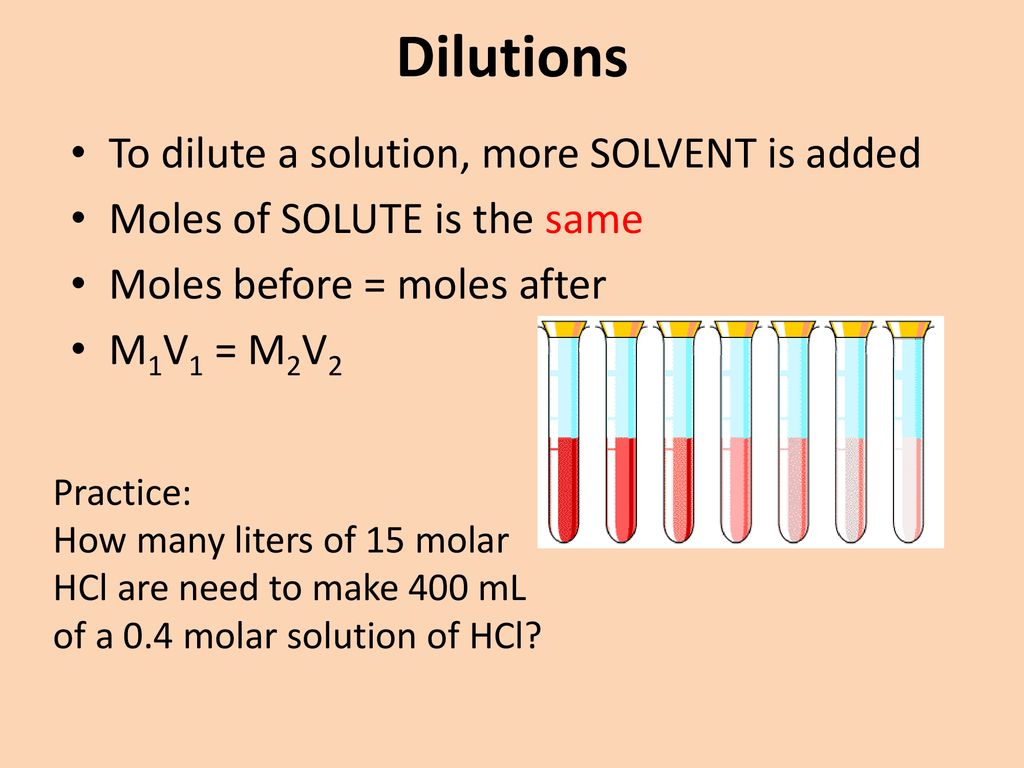
Dilutions Practice Problems M1V1=M2V2 . M1v1 is the concentration and volume of the stock solution. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. To dilute a liquid stock solution, the following formula is used: Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To solve these problems, use m1v1 = m2v2. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. 3) explain the difference between an endpoint and equivalence point in a titration. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to.
from slideplayer.com
To solve these problems, use m1v1 = m2v2. M1v1 is the concentration and volume of the stock solution. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. To dilute a liquid stock solution, the following formula is used: Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an endpoint and equivalence point in a titration. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?.
Mixtures (Solutions) Heterogeneous Homogeneous Solution Heterogeneous
Dilutions Practice Problems M1V1=M2V2 Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. M1v1 is the concentration and volume of the stock solution. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 3) explain the difference between an endpoint and equivalence point in a titration. To solve these problems, use m1v1 = m2v2. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To dilute a liquid stock solution, the following formula is used: Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to.
From www.docsity.com
Dilution Problems Worksheet (M1V1 = M2V2) Monografías, Ensayos Dilutions Practice Problems M1V1=M2V2 Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. To solve these problems, use m1v1 = m2v2. M1v1 is the concentration and volume of the stock solution. How much. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions (Chapter 14). ppt download Dilutions Practice Problems M1V1=M2V2 To solve these problems, use m1v1 = m2v2. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an endpoint and equivalence point in a titration. 🧪 dilution problems are discussed. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Mixtures (Solutions) Heterogeneous Homogeneous Solution Heterogeneous Dilutions Practice Problems M1V1=M2V2 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. M1v1 is the concentration and volume of the stock solution. Dilution problems worksheet (m 1. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
You will turn in this assignment. ppt download Dilutions Practice Problems M1V1=M2V2 Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. How much of a 15.0 m stock. Dilutions Practice Problems M1V1=M2V2.
From ar.inspiredpencil.com
M1v1 M2v2 Chemistry Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions and Molarity ppt download Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To dilute a liquid stock solution, the following formula is used: M1v1 is the concentration and volume of the stock solution. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions. ppt download Dilutions Practice Problems M1V1=M2V2 Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. To solve these problems, use m1v1 = m2v2. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions. ppt download Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. To solve these problems, use m1v1 = m2v2. M1v1 is the concentration and volume of the stock solution. To dilute a liquid stock solution, the following formula is used: M1 and v1 are the molarity. Dilutions Practice Problems M1V1=M2V2.
From lessoncampusdebby.z22.web.core.windows.net
Dilution Problems Worksheet M1v1 M2v2 Answer Key Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. To dilute a liquid stock solution, the following formula is used: 3) explain the difference between an endpoint and equivalence point in a titration. M1v1 is the. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Stoichiometry Quantitative Information About Chemical Reactions ppt Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. To solve these problems, use m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. 3) explain the difference between an endpoint and equivalence point in a titration. 🧪 dilution problems are discussed using. Dilutions Practice Problems M1V1=M2V2.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6750404 Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. To. Dilutions Practice Problems M1V1=M2V2.
From www.studocu.com
Conversions and Dilutions Practice Problems Answer Key Practice Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. M1v1 is the concentration and volume of the stock solution. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an endpoint and. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Stoichiometry and molarity practice ppt download Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. 3) explain the difference between an endpoint and equivalence point in a titration. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution.. Dilutions Practice Problems M1V1=M2V2.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry Dilutions Practice Problems M1V1=M2V2 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. 3) explain the difference between an endpoint and. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilutions Practice Problems M1V1=M2V2 M1v1 is the concentration and volume of the stock solution. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. How much of. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Chapters 15 and 16 Unique properties of water Solution formation ppt Dilutions Practice Problems M1V1=M2V2 Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To dilute a liquid stock solution, the following formula is used: Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how. Dilutions Practice Problems M1V1=M2V2.
From ar.inspiredpencil.com
M1v1=m2v2 Dilutions Practice Problems M1V1=M2V2 M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. 3) explain the difference between an endpoint and equivalence point in a titration. To solve these problems, use m1v1 = m2v2. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m. Dilutions Practice Problems M1V1=M2V2.
From www.slideserve.com
PPT Making Molar Solutions PowerPoint Presentation, free download Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an endpoint and equivalence point in a titration. M1 and v1 are the. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions Chapter ppt download Dilutions Practice Problems M1V1=M2V2 3) explain the difference between an endpoint and equivalence point in a titration. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume. Dilutions Practice Problems M1V1=M2V2.
From www.studocu.com
Dilution Formula = M1V1 = M2V22 CHE 115 Trial Number Concentration Dilutions Practice Problems M1V1=M2V2 Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. M1v1 is the concentration and volume of the stock solution. To dilute a liquid stock solution, the following formula is used: Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. How much of a 15.0 m stock solution do you need to prepare 250. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube Dilutions Practice Problems M1V1=M2V2 To solve these problems, use m1v1 = m2v2. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. How much of a 15.0 m. Dilutions Practice Problems M1V1=M2V2.
From www.slideserve.com
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061 Dilutions Practice Problems M1V1=M2V2 M1v1 is the concentration and volume of the stock solution. 3) explain the difference between an endpoint and equivalence point in a titration. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry |. Dilutions Practice Problems M1V1=M2V2.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To dilute a liquid stock solution, the following formula is used: Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. 3) explain the difference between an endpoint and equivalence point in a titration. To solve these. Dilutions Practice Problems M1V1=M2V2.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3927909 Dilutions Practice Problems M1V1=M2V2 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. To solve these problems, use m1v1 = m2v2. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. How much of a 15.0 m stock. Dilutions Practice Problems M1V1=M2V2.
From lessoncampusdebby.z22.web.core.windows.net
Dilution Problems Worksheet M1v1 M2v2 Answer Key Dilutions Practice Problems M1V1=M2V2 To dilute a liquid stock solution, the following formula is used: 3) explain the difference between an endpoint and equivalence point in a titration. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. M1v1 is the concentration and volume of the stock solution.. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions How can one differentiate between saturated, unsaturated, and Dilutions Practice Problems M1V1=M2V2 Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. 3) explain the difference between an endpoint and equivalence point in a titration. To solve these problems, use m1v1 = m2v2. M1 and v1 are the. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilutions Practice Problems M1V1=M2V2 3) explain the difference between an endpoint and equivalence point in a titration. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. M1v1 is. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Dilution Of Solution M1V1 = M2V2 Chemistry Catalyst YouTube Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. M1v1 is the concentration and volume of the stock solution. To dilute a liquid stock solution, the following formula is used: Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 3) explain the difference between. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Quick video Dilution problems M1V1=M2V2 YouTube Dilutions Practice Problems M1V1=M2V2 3) explain the difference between an endpoint and equivalence point in a titration. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. To solve these problems, use m1v1 = m2v2. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and volume of the diluted solution.. Dilutions Practice Problems M1V1=M2V2.
From prntbl.concejomunicipaldechinu.gov.co
Dilution Problems Worksheet M1v1 M2v2 Answer Key prntbl Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. To solve these problems, use m1v1 = m2v2. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are the molarity and. Dilutions Practice Problems M1V1=M2V2.
From studylib.net
Key DilutionsWorksheet Dilutions Practice Problems M1V1=M2V2 3) explain the difference between an endpoint and equivalence point in a titration. To solve these problems, use m1v1 = m2v2. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. M1 and v1 are the molarity and volume of the concentrated stock solution, and m2 and v2 are. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Stoichiometry Practice Problems Dilutions M1V1 = M2V2 General Dilutions Practice Problems M1V1=M2V2 Let's practice calculating a dilution for solutions using m1v1 = m2v2!please email. M1v1 is the concentration and volume of the stock solution. To dilute a liquid stock solution, the following formula is used: To solve these problems, use m1v1 = m2v2. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong. Dilutions Practice Problems M1V1=M2V2.
From slideplayer.com
Solutions. ppt download Dilutions Practice Problems M1V1=M2V2 How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. 3) explain the difference between an endpoint and equivalence point in a titration. Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. To dilute a liquid stock. Dilutions Practice Problems M1V1=M2V2.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilutions Practice Problems M1V1=M2V2 To dilute a liquid stock solution, the following formula is used: M1v1 is the concentration and volume of the stock solution. 🧪 dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume. To solve these problems, use m1v1 = m2v2. How much of a 15.0 m stock solution do you need to. Dilutions Practice Problems M1V1=M2V2.
From www.youtube.com
Practice with Dilutions Chemistry Dilutions M1V1 = M2V2 (60) YouTube Dilutions Practice Problems M1V1=M2V2 To dilute a liquid stock solution, the following formula is used: Dilution equation | m1v1=m2v2 | practice problem #1 | solution chemistry | chemistry | how to dilute a strong acid/base to. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. To solve these problems, use m1v1 =. Dilutions Practice Problems M1V1=M2V2.